Page 305 - Physical Chemistry
P. 305
lev38627_ch09.qxd 3/14/08 1:31 PM Page 286
286
Chapter 9 Acetone chloroform
Solutions
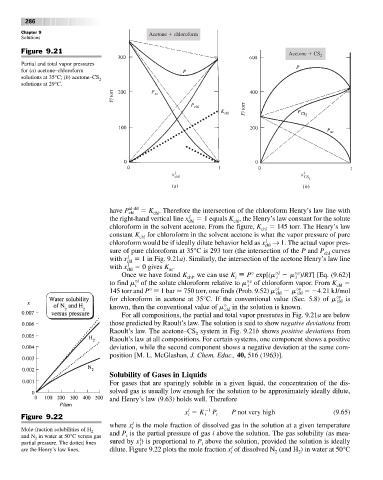
Figure 9.21 Acetone CS 2
Partial and total vapor pressures
for (a) acetone–chloroform
solutions at 35°C; (b) acetone–CS 2
solutions at 29°C.
x l x l
chl CS
2
have P id-dil K . Therefore the intersection of the chloroform Henry’s law line with
chl
chl
l
the right-hand vertical line x chl 1 equals K , the Henry’s law constant for the solute
chl
chloroform in the solvent acetone. From the figure, K chl 145 torr. The Henry’s law
constant K chl for chloroform in the solvent acetone is what the vapor pressure of pure
l
chloroform would be if ideally dilute behavior held as x chl → 1. The actual vapor pres-
sure of pure chloroform at 35°C is 293 torr (the intersection of the P and P chl curves
l
with x chl 1 in Fig. 9.21a). Similarly, the intersection of the acetone Henry’s law line
l
with x chl 0 gives K .
ac
v
l
Once we have found K , we can use K P°exp[(m° m° )/RT] [Eq. (9.62)]
i
chl
i
i
l
v
to find m° of the solute chloroform relative to m° of chloroform vapor. From K chl
i
i
v
l
145 torr and P° 1 bar 750 torr, one finds (Prob. 9.52) m° m° chl 4.21 kJ/mol
chl
v
for chloroform in acetone at 35°C. If the conventional value (Sec. 5.8) of m° chl is
known, then the conventional value of m° in the solution is known.
chl
For all compositions, the partial and total vapor pressures in Fig. 9.21a are below
those predicted by Raoult’s law. The solution is said to show negative deviations from
Raoult’s law. The acetone–CS system in Fig. 9.21b shows positive deviations from
2
Raoult’s law at all compositions. For certain systems, one component shows a positive
deviation, while the second component shows a negative deviation at the same com-
position [M. L. McGlashan, J. Chem. Educ., 40, 516 (1963)].
Solubility of Gases in Liquids
For gases that are sparingly soluble in a given liquid, the concentration of the dis-
solved gas is usually low enough for the solution to be approximately ideally dilute,
and Henry’s law (9.63) holds well. Therefore
l
x K 1 P P not very high (9.65)
i
i
i
Figure 9.22
l
where x is the mole fraction of dissolved gas in the solution at a given temperature
Mole-fraction solubilities of H 2 i
i
and N in water at 50°C versus gas and P is the partial pressure of gas i above the solution. The gas solubility (as mea-
2
l
partial pressure. The dotted lines sured by x ) is proportional to P above the solution, provided the solution is ideally
i
i
l
are the Henry’s law lines. dilute. Figure 9.22 plots the mole fraction x of dissolved N (and H ) in water at 50°C
i 2 2