Page 332 - Physical Chemistry
P. 332
lev38627_ch10.qxd 3/14/08 1:07 PM Page 313
313
Equation (10.65) is called the Debye–Hückel limiting law, since it is valid only in the Section 10.7
limit of infinite dilution. (Actually, most of the laws of science are limiting laws.) The Debye–Hückel Theory of
Electrolyte Solutions
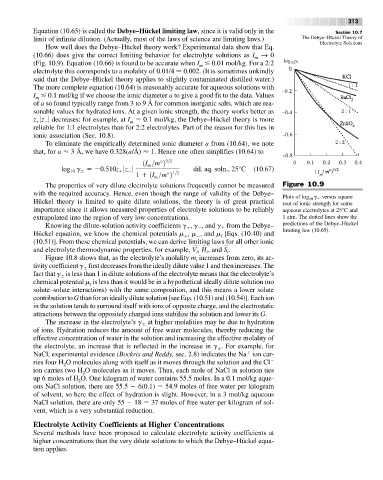
How well does the Debye–Hückel theory work? Experimental data show that Eq.
(10.66) does give the correct limiting behavior for electrolyte solutions as I → 0
m
(Fig. 10.9). Equation (10.66) is found to be accurate when I 0.01 mol/kg. For a 2:2 log 10 g
m
electrolyte this corresponds to a molality of 0.01/4 0.002. (It is sometimes unkindly
said that the Debye–Hückel theory applies to slightly contaminated distilled water.)
The more complete equation (10.64) is reasonably accurate for aqueous solutions with
I 0.1 mol/kg if we choose the ionic diameter a to give a good fit to the data. Values
m
of a so found typically range from 3 to 9 Å for common inorganic salts, which are rea-
sonable values for hydrated ions. At a given ionic strength, the theory works better as
z z decreases; for example, at I 0.1 mol/kg, the Debye–Hückel theory is more
m
reliable for 1:1 electrolytes than for 2:2 electrolytes. Part of the reason for this lies in
ionic association (Sec. 10.8).
To eliminate the empirically determined ionic diameter a from (10.64), we note
that, for a 3 Å, we have 0.328(a/Å) 1. Hence one often simplifies (10.64) to
1I >m°2 1>2
m
log g 0.510z 0z 0 dil. aq. soln., 25°C (10.67)
10
1 1I >m°2 1>2
m
The properties of very dilute electrolyte solutions frequently cannot be measured Figure 10.9
with the required accuracy. Hence, even though the range of validity of the Debye–
Plots of log g versus square
10
Hückel theory is limited to quite dilute solutions, the theory is of great practical root of ionic strength for some
importance since it allows measured properties of electrolyte solutions to be reliably aqueous electrolytes at 25°C and
extrapolated into the region of very low concentrations. 1 atm. The dotted lines show the
Knowing the dilute-solution activity coefficients g , g , and g from the Debye– predictions of the Debye–Hückel
Hückel equation, we know the chemical potentials m , m , and m [Eqs. (10.40) and limiting law (10.65).
i
(10.51)]. From these chemical potentials, we can derive limiting laws for all other ionic
and electrolyte thermodynamic properties, for example, V , i H , and S .
i
i
Figure 10.8 shows that, as the electrolyte’s molality m increases from zero, its ac-
i
tivity coefficient g first decreases from the ideally dilute value 1 and then increases. The
fact that g is less than 1 in dilute solutions of the electrolyte means that the electrolyte’s
chemical potential m is less than it would be in a hypothetical ideally dilute solution (no
i
solute–solute interactions) with the same composition, and this means a lower solute
contribution to G than for an ideally dilute solution [see Eqs. (10.51) and (10.54)]. Each ion
in the solution tends to surround itself with ions of opposite charge, and the electrostatic
attractions between the oppositely charged ions stabilize the solution and lower its G.
The increase in the electrolyte’s g at higher molalities may be due to hydration
of ions. Hydration reduces the amount of free water molecules, thereby reducing the
effective concentration of water in the solution and increasing the effective molality of
the electrolyte, an increase that is reflected in the increase in g . For example, for
NaCl, experimental evidence (Bockris and Reddy, sec. 2.8) indicates the Na ion car-
ries four H O molecules along with itself as it moves through the solution and the Cl
2
ion carries two H O molecules as it moves. Thus, each mole of NaCl in solution ties
2
up 6 moles of H O. One kilogram of water contains 55.5 moles. In a 0.1 mol/kg aque-
2
ous NaCl solution, there are 55.5 6(0.1) 54.9 moles of free water per kilogram
of solvent, so here the effect of hydration is slight. However, in a 3 mol/kg aqueous
NaCl solution, there are only 55 18 37 moles of free water per kilogram of sol-
vent, which is a very substantial reduction.
Electrolyte Activity Coefficients at Higher Concentrations
Several methods have been proposed to calculate electrolyte activity coefficients at
higher concentrations than the very dilute solutions to which the Debye–Hückel equa-
tion applies.