Page 112 - Plant design and economics for chemical engineers
P. 112
88 PLANT DESlGN AND ECONOMICS FOR CHEMICAL ENGINEERS
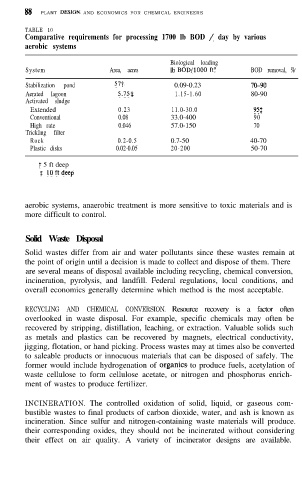
TABLE 10
Comparative requirements for processing 1700 lb BOD / day by various
aerobic systems
Biological loading
System Area, acres lb BOD/lOOO ft3 BOD removal, %
Stabilization pond 57t 0.09-0.23 70-90
Aerated lagoon 5.75s 1.15-1.60 80-90
Activated sludge
Extended 0.23 11.0-30.0 95:
Conventional 0.08 33.0-400 90
High rate 0.046 57.0-150 70
Trickling filter
Rock 0.2-0.5 0.7-50 40-70
Plastic disks 0.02-0.05 20-200 50-70
t 5 ft deep
$ loftdeep
aerobic systems, anaerobic treatment is more sensitive to toxic materials and is
more difficult to control.
Solid Waste Disposal
Solid wastes differ from air and water pollutants since these wastes remain at
the point of origin until a decision is made to collect and dispose of them. There
are several means of disposal available including recycling, chemical conversion,
incineration, pyrolysis, and landfill. Federal regulations, local conditions, and
overall economics generally determine which method is the most acceptable.
RECYCLING AND CHEMICAL CONVERSION. Resource recovery is a factor often
overlooked in waste disposal. For example, specific chemicals may often be
recovered by stripping, distillation, leaching, or extraction. Valuable solids such
as metals and plastics can be recovered by magnets, electrical conductivity,
jigging, flotation, or hand picking. Process wastes may at times also be converted
to saleable products or innocuous materials that can be disposed of safely. The
former would include hydrogenation of organics to produce fuels, acetylation of
waste cellulose to form cellulose acetate, or nitrogen and phosphorus enrich-
ment of wastes to produce fertilizer.
INCINERATION. The controlled oxidation of solid, liquid, or gaseous com-
bustible wastes to final products of carbon dioxide, water, and ash is known as
incineration. Since sulfur and nitrogen-containing waste materials will produce.
their corresponding oxides, they should not be incinerated without considering
their effect on air quality. A variety of incinerator designs are available.