Page 139 - Plant design and economics for chemical engineers
P. 139
114 PLANT DESIGN AND ECONOMICS FOR CHEMICAL ENGINEERS
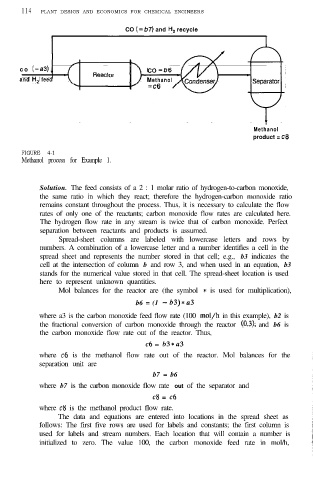
CO (=b7) and H, recycle
c o (=a3) , CO =b6
and H, feed Methanol
=c6
Methanol
Methanol
product = c8
product =
c8
FIGURE 4-1
Methanol process for Example 1.
Solution. The feed consists of a 2 : 1 molar ratio of hydrogen-to-carbon monoxide,
the same ratio in which they react; therefore the hydrogen-carbon monoxide ratio
remains constant throughout the process. Thus, it is necessary to calculate the flow
rates of only one of the reactants; carbon monoxide flow rates are calculated here.
The hydrogen flow rate in any stream is twice that of carbon monoxide. Perfect
separation between reactants and products is assumed.
Spread-sheet columns are labeled with lowercase letters and rows by
numbers. A combination of a lowercase letter and a number identifies a cell in the
spread sheet and represents the number stored in that cell; e.g., b3 indicates the
cell at the intersection of column b and row 3, and when used in an equation, b3
stands for the numerical value stored in that cell. The spread-sheet location is used
here to represent unknown quantities.
Mol balances for the reactor are (the symbol * is used for multiplication),
b6 = (1 - b3)*n3
where a3 is the carbon monoxide feed flow rate (100 mol/h in this example), b2 is
the fractional conversion of carbon monoxide through the reactor (0.3), and b6 is
the carbon monoxide flow rate out of the reactor. Thus,
c6 = b3*a3
where c6 is the methanol flow rate out of the reactor. Mol balances for the
separation unit are
b7 = b6
where b7 is the carbon monoxide flow rate out of the separator and
c8 = c6
where c8 is the methanol product flow rate.
The data and equations are entered into locations in the spread sheet as
follows: The first five rows are used for labels and constants; the first column is
used for labels and stream numbers. Each location that will contain a number is
initialized to zero. The value 100, the carbon monoxide feed rate in mol/h,