Page 113 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 113
Nanoclay and polymer-based nanocomposites: Materials for energy efficiency 89
O
OH
Al
Si
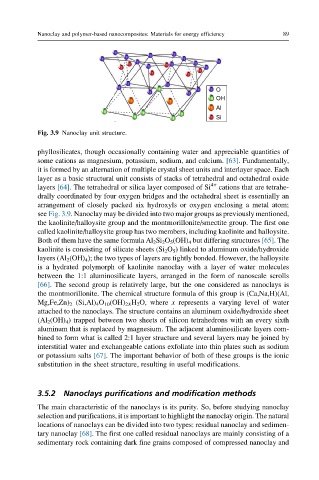
Fig. 3.9 Nanoclay unit structure.
phyllosilicates, though occasionally containing water and appreciable quantities of
some cations as magnesium, potassium, sodium, and calcium. [63]. Fundamentally,
it is formed by an alternation of multiple crystal sheet units and interlayer space. Each
layer as a basic structural unit consists of stacks of tetrahedral and octahedral oxide
layers [64]. The tetrahedral or silica layer composed of Si 4+ cations that are tetrahe-
drally coordinated by four oxygen bridges and the octahedral sheet is essentially an
arrangement of closely packed six hydroxyls or oxygen enclosing a metal atom;
see Fig. 3.9. Nanoclay may be divided into two major groups as previously mentioned,
the kaolinite/halloysite group and the montmorillonite/smectite group. The first one
called kaolinite/halloysite group has two members, including kaolinite and halloysite.
Both of them have the same formula Al 2 Si 2 O 5 (OH) 4 but differing structures [65]. The
kaolinite is consisting of silicate sheets (Si 2 O 5 ) linked to aluminum oxide/hydroxide
layers (Al 2 (OH) 4 ); the two types of layers are tightly bonded. However, the halloysite
is a hydrated polymorph of kaolinite nanoclay with a layer of water molecules
between the 1:1 aluminosilicate layers, arranged in the form of nanoscale scrolls
[66]. The second group is relatively large, but the one considered as nanoclays is
the montmorillonite. The chemical structure formula of this group is (Ca,Na,H)(Al,
Mg,Fe,Zn) 2 (Si,Al) 4 O 10 (OH) 2X H 2 O, where x represents a varying level of water
attached to the nanoclays. The structure contains an aluminum oxide/hydroxide sheet
(Al 2 (OH) 4 ) trapped between two sheets of silicon tetrahedrons with an every sixth
aluminum that is replaced by magnesium. The adjacent aluminosilicate layers com-
bined to form what is called 2:1 layer structure and several layers may be joined by
interstitial water and exchangeable cations exfoliate into thin plates such as sodium
or potassium salts [67]. The important behavior of both of these groups is the ionic
substitution in the sheet structure, resulting in useful modifications.
3.5.2 Nanoclays purifications and modification methods
The main characteristic of the nanoclays is its purity. So, before studying nanoclay
selection and purifications, it is important to highlight the nanoclay origin. The natural
locations of nanoclays can be divided into two types: residual nanoclay and sedimen-
tary nanoclay [68]. The first one called residual nanoclays are mainly consisting of a
sedimentary rock containing dark fine grains composed of compressed nanoclay and