Page 278 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 278
250 Polymer-based Nanocomposites for Energy and Environmental Applications
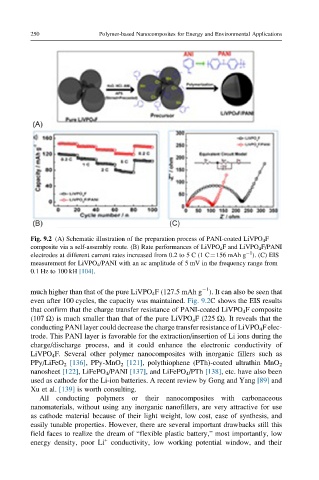
Fig. 9.2 (A) Schematic illustration of the preparation process of PANI-coated LiVPO 4 F
composite via a self-assembly route. (B) Rate performances of LiVPO 4 F and LiVPO 4 F/PANI
1
electrodes at different current rates increased from 0.2 to 5 C (1 C¼156 mAh g ). (C) EIS
measurement for LiVPO 4 /PANI with an ac amplitude of 5 mV in the frequency range from
0.1 Hz to 100 kH [104].
1
much higher than that of the pure LiVPO 4 F (127.5 mAh g ). It can also be seen that
even after 100 cycles, the capacity was maintained. Fig. 9.2C shows the EIS results
that confirm that the charge transfer resistance of PANI-coated LiVPO 4 F composite
(107 Ω) is much smaller than that of the pure LiVPO 4 F (225 Ω). It reveals that the
conducting PANI layer could decrease the charge transfer resistance of LiVPO 4 F elec-
trode. This PANI layer is favorable for the extraction/insertion of Li ions during the
charge/discharge process, and it could enhance the electronic conductivity of
LiVPO 4 F. Several other polymer nanocomposites with inorganic fillers such as
PPy/LiFeO 2 [136], PPy-MnO 2 [121], polythiophene (PTh)-coated ultrathin MnO 2
nanosheet [122], LiFePO 4 /PANI [137], and LiFePO 4 /PTh [138], etc. have also been
used as cathode for the Li-ion batteries. A recent review by Gong and Yang [89] and
Xu et al. [139] is worth consulting.
All conducting polymers or their nanocomposites with carbonaceous
nanomaterials, without using any inorganic nanofillers, are very attractive for use
as cathode material because of their light weight, low cost, ease of synthesis, and
easily tunable properties. However, there are several important drawbacks still this
field faces to realize the dream of “flexible plastic battery,” most importantly, low
+
energy density, poor Li conductivity, low working potential window, and their