Page 280 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 280
252 Polymer-based Nanocomposites for Energy and Environmental Applications
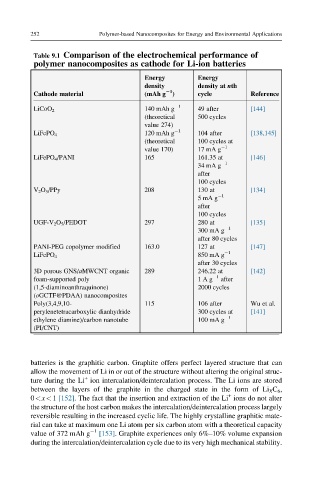
Table 9.1 Comparison of the electrochemical performance of
polymer nanocomposites as cathode for Li-ion batteries
Energy Energy
density density at nth
Cathode material (mAh g 21 ) cycle Reference
140 mAh g 1 49 after [144]
LiCoO 2
(theoretical 500 cycles
value 274)
120 mAh g 1 104 after [138,145]
LiFePO 4
(theoretical 100 cycles at
value 170) 17 mA g 1
LiFePO 4 /PANI 165 161.35 at [146]
34 mA g 1
after
100 cycles
V 2 O 5 /PPy 208 130 at [134]
5mAg 1
after
100 cycles
UGF-V 2 O 5 /PEDOT 297 280 at [135]
300 mA g 1
after 80 cycles
PANI-PEG copolymer modified 163.0 127 at [147]
850 mA g 1
LiFePO 4
after 30 cycles
3D porous GNS/aMWCNT organic 289 246.22 at [142]
foam-supported poly 1Ag 1 after
(1,5-diaminoanthraquinone) 2000 cycles
(oGCTF@PDAA) nanocomposites
Poly(3,4,9,10- 115 106 after Wu et al.
perylenetetracarboxylic dianhydride 300 cycles at [141]
ethylene diamine)/carbon nanotube 100 mA g 1
(PI/CNT)
batteries is the graphitic carbon. Graphite offers perfect layered structure that can
allow the movement of Li in or out of the structure without altering the original struc-
+
ture during the Li ion intercalation/deintercalation process. The Li ions are stored
between the layers of the graphite in the charged state in the form of Li X C 6 ,
+
0<x<1 [152]. The fact that the insertion and extraction of the Li ions do not alter
the structure of the host carbon makes the intercalation/deintercalation process largely
reversible resulting in the increased cyclic life. The highly crystalline graphitic mate-
rial can take at maximum one Li atom per six carbon atom with a theoretical capacity
value of 372 mAh g 1 [153]. Graphite experiences only 6%–10% volume expansion
during the intercalation/deintercalation cycle due to its very high mechanical stability.