Page 489 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 489
442 Polymer-based Nanocomposites for Energy and Environmental Applications
methods of eliminating heavy metals from solutions [6]. Cellulose, which is found in
huge quantity in nature and constitutes the main part of plant fibers, is responsible for
the rigidity of the plant.
Materials based on cellulosic can be acquired and used as low-cost adsorbents, and
their presentation to eliminate heavy metal ions can be changed by chemical treat-
ment. Generally, chemical changes in cellulose materials lead to higher adsorption
capacities than unchanged forms. Furthermore, nanoadsorbents provide significant
improvements with their tremendously high specific surface area linked with sorption
sites and short intraparticle diffusion distance leading to fast kinetics [7]. For instance,
the arsenic adsorption capacity onto nanomagnetite increased >100 times, when its
particle size decreased from 300 to 11 nm [8]. Other advantages of the properties
of nanoadsorbents include enhanced redox, superparamagnetism, and photocatalytic
properties depending on the type of nanosized adsorbents [7]. Table 16.1 lists some of
the nanomaterials studied for water purification and the involved mechanisms. It may
be noted that some of these nanomaterials target heavy metal ions. However, it is
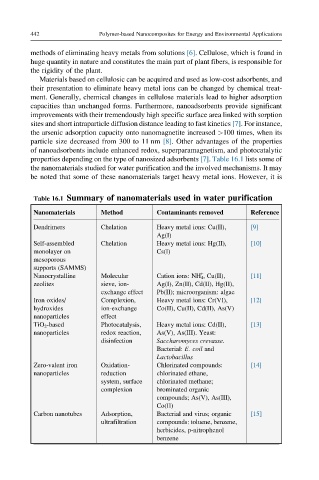
Table 16.1 Summary of nanomaterials used in water purification
Nanomaterials Method Contaminants removed Reference
Dendrimers Chelation Heavy metal ions: Cu(II), [9]
Ag(I)
Self-assembled Chelation Heavy metal ions: Hg(II), [10]
monolayer on Cs(I)
mesoporous
supports (SAMMS)
+
Nanocrystalline Molecular Cation ions: NH 4 , Cu(II), [11]
zeolites sieve, ion- Ag(I), Zn(II), Cd(II), Hg(II),
exchange effect Pb(II); microorganism: algae
Iron oxides/ Complexion, Heavy metal ions: Cr(VI), [12]
hydroxides ion-exchange Co(II), Cu(II), Cd(II), As(V)
nanoparticles effect
TiO 2 -based Photocatalysis, Heavy metal ions: Cd(II), [13]
nanoparticles redox reaction, As(V), As(III). Yeast:
disinfection Saccharomyces crevasse.
Bacterial: E. coil and
Lactobacillus
Zero-valent iron Oxidation- Chlorinated compounds: [14]
nanoparticles reduction chlorinated ethane,
system, surface chlorinated methane;
complexion brominated organic
compounds; As(V), As(III),
Co(II)
Carbon nanotubes Adsorption, Bacterial and virus; organic [15]
ultrafiltration compounds: toluene, benzene,
herbicides, p-nitrophenol
benzene