Page 514 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 514
Polypyrrole-based nanocomposite adsorbents 467
H H +
N N
A –
N
H
(A) n
H H
N N
N
H
(B) n
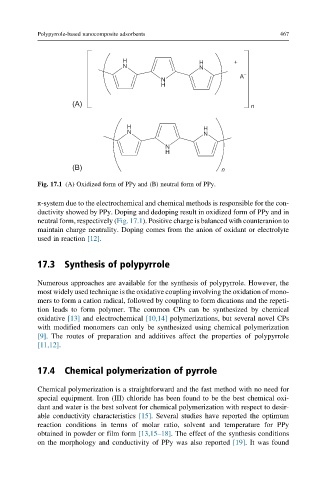
Fig. 17.1 (A) Oxidized form of PPy and (B) neutral form of PPy.
π-system due to the electrochemical and chemical methods is responsible for the con-
ductivity showed by PPy. Doping and dedoping result in oxidized form of PPy and in
neutral form, respectively (Fig. 17.1). Positive charge is balanced with counteranion to
maintain charge neutrality. Doping comes from the anion of oxidant or electrolyte
used in reaction [12].
17.3 Synthesis of polypyrrole
Numerous approaches are available for the synthesis of polypyrrole. However, the
most widely used technique is the oxidative coupling involving the oxidation of mono-
mers to form a cation radical, followed by coupling to form dications and the repeti-
tion leads to form polymer. The common CPs can be synthesized by chemical
oxidative [13] and electrochemical [10,14] polymerizations, but several novel CPs
with modified monomers can only be synthesized using chemical polymerization
[9]. The routes of preparation and additives affect the properties of polypyrrole
[11,12].
17.4 Chemical polymerization of pyrrole
Chemical polymerization is a straightforward and the fast method with no need for
special equipment. Iron (III) chloride has been found to be the best chemical oxi-
dant and water is the best solvent for chemical polymerization with respect to desir-
able conductivity characteristics [15]. Several studies have reported the optimum
reaction conditions in terms of molar ratio, solvent and temperature for PPy
obtained in powder or film form [13,15–18]. The effect of the synthesis conditions
on the morphology and conductivity of PPy was also reported [19]. It was found