Page 515 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 515
468 Polymer-based Nanocomposites for Energy and Environmental Applications
+
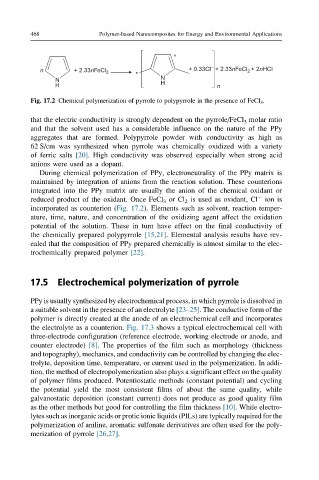
n + 2.33nFeCl 3 + 0.33Cl – + 2.33nFeCl + 2nHCl
2
*
N N
H H n
Fig. 17.2 Chemical polymerization of pyrrole to polypyrrole in the presence of FeCl 3 .
that the electric conductivity is strongly dependent on the pyrrole/FeCl 3 molar ratio
and that the solvent used has a considerable influence on the nature of the PPy
aggregates that are formed. Polypyrrole powder with conductivity as high as
62 S/cm was synthesized when pyrrole was chemically oxidized with a variety
of ferric salts [20]. High conductivity was observed especially when strong acid
anions were used as a dopant.
During chemical polymerization of PPy, electroneutrality of the PPy matrix is
maintained by integration of anions from the reaction solution. These counterions
integrated into the PPy matrix are usually the anion of the chemical oxidant or
reduced product of the oxidant. Once FeCl 3 or Cl 2 is used as oxidant, Cl ion is
incorporated as counterion (Fig. 17.2). Elements such as solvent, reaction temper-
ature, time, nature, and concentration of the oxidizing agent affect the oxidation
potential of the solution. These in turn have effect on the final conductivity of
the chemically prepared polypyrrole [15,21]. Elemental analysis results have rev-
ealed that the composition of PPy prepared chemically is almost similar to the elec-
trochemically prepared polymer [22].
17.5 Electrochemical polymerization of pyrrole
PPy is usually synthesized by electrochemical process, in which pyrrole is dissolved in
a suitable solvent in the presence of an electrolyte [23–25]. The conductive form of the
polymer is directly created at the anode of an electrochemical cell and incorporates
the electrolyte as a counterion. Fig. 17.3 shows a typical electrochemical cell with
three-electrode configuration (reference electrode, working electrode or anode, and
counter electrode) [8]. The properties of the film such as morphology (thickness
and topography), mechanics, and conductivity can be controlled by changing the elec-
trolyte, deposition time, temperature, or current used in the polymerization. In addi-
tion, the method of electropolymerization also plays a significant effect on the quality
of polymer films produced. Potentiostatic methods (constant potential) and cycling
the potential yield the most consistent films of about the same quality, while
galvanostatic deposition (constant current) does not produce as good quality film
as the other methods but good for controlling the film thickness [10]. While electro-
lytes such as inorganic acids or protic ionic liquids (PILs) are typically required for the
polymerization of aniline, aromatic sulfonate derivatives are often used for the poly-
merization of pyrrole [26,27].