Page 562 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 562
Hybrid materials based on polymer nanocomposites for environmental applications 515
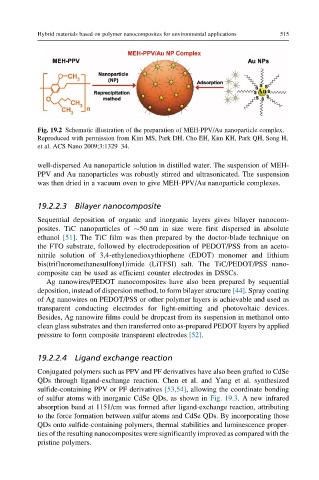
Fig. 19.2 Schematic illustration of the preparation of MEH-PPV/Au nanoparticle complex.
Reproduced with permission from Kim MS, Park DH, Cho EH, Kim KH, Park QH, Song H,
et al. ACS Nano 2009;3:1329–34.
well-dispersed Au nanoparticle solution in distilled water. The suspension of MEH-
PPV and Au nanoparticles was robustly stirred and ultrasonicated. The suspension
was then dried in a vacuum oven to give MEH-PPV/Au nanoparticle complexes.
19.2.2.3 Bilayer nanocomposite
Sequential deposition of organic and inorganic layers gives bilayer nanocom-
posites. TiC nanoparticles of 50 nm in size were first dispersed in absolute
ethanol [51]. The TiC film was then prepared by the doctor-blade technique on
the FTO substrate, followed by electrodeposition of PEDOT/PSS from an aceto-
nitrile solution of 3,4-ethylenedioxythiophene (EDOT) monomer and lithium
bis(trifluoromethanesulfonyl)imide (LiTFSI) salt. The TiC/PEDOT/PSS nano-
composite can be used as efficient counter electrodes in DSSCs.
Ag nanowires/PEDOT nanocomposites have also been prepared by sequential
deposition, instead of dispersion method, to form bilayer structure [44]. Spray coating
of Ag nanowires on PEDOT/PSS or other polymer layers is achievable and used as
transparent conducting electrodes for light-emitting and photovoltaic devices.
Besides, Ag nanowire films could be dropcast from its suspension in methanol onto
clean glass substrates and then transferred onto as-prepared PEDOT layers by applied
pressure to form composite transparent electrodes [52].
19.2.2.4 Ligand exchange reaction
Conjugated polymers such as PPV and PF derivatives have also been grafted to CdSe
QDs through ligand-exchange reaction. Chen et al. and Yang et al. synthesized
sulfide-containing PPV or PF derivatives [53,54], allowing the coordinate bonding
of sulfur atoms with inorganic CdSe QDs, as shown in Fig. 19.3. A new infrared
absorption band at 1151/cm was formed after ligand-exchange reaction, attributing
to the force formation between sulfur atoms and CdSe QDs. By incorporating those
QDs onto sulfide-containing polymers, thermal stabilities and luminescence proper-
ties of the resulting nanocomposites were significantly improved as compared with the
pristine polymers.