Page 651 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 651
Recent advances in polyaniline-based nanocomposites as potential adsorbents for trace metal ions 601
incorporated ligand. These chelating groups provide a negative residual charge to the
surface of composites. Thus, depending on the solution pH, the surface atoms hold a
lone pair of electron responsible for interaction with metal ions. Compared with other
adsorbents, the utilization of polyaniline nanocomposites may offer several advan-
tages. First, organic polymeric part of the composite provides mechanical and chem-
ical stability, whereas the inorganic structure part supports the metal ion complexation
regarding its selectivity for some particular metal ions and thermal stability and also
increases the electric conductivity. In addition to these characteristics, the hybrid
nanomaterials were considered as new composite materials that exhibit very different
properties from their original components, that is, organic polymer and inorganic
materials especially in the case of molecular level hybrids. Thus, the synthesis of poly-
aniline nanocomposites has received a great deal of attention because it provided new
materials with special mechanical, chemical, electrochemical, and optical as well as
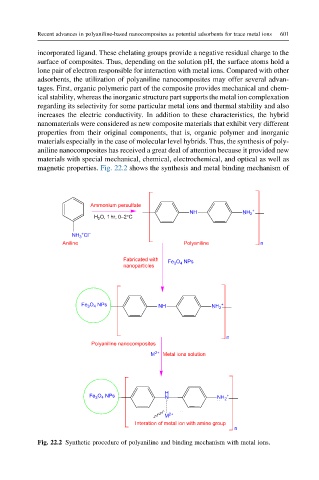
magnetic properties. Fig. 22.2 shows the synthesis and metal binding mechanism of
Ammonium persulfate
NH NH 2 +
H 2 O, 1 hr, 0−2°C
+ −
NH 3 Cl
Aniline Polyaniline n
Fabricated with Fe 3 O 4 NPs
nanoparticles
Fe 3 O 4 NPs NH NH 2 +
n
Polyaniline nanocomposites
M 2+ Metal ions solution
H
Fe 3 O 4 NPs N NH 2 +
M 2+
Interation of metal ion with amine group
n
Fig. 22.2 Synthetic procedure of polyaniline and binding mechanism with metal ions.