Page 56 - Practical Well Planning and Drilling Manual
P. 56
Section 1 revised 11/00/bc 1/17/01 2:55 PM Page 32
[ ]
1.3.5 Well Design
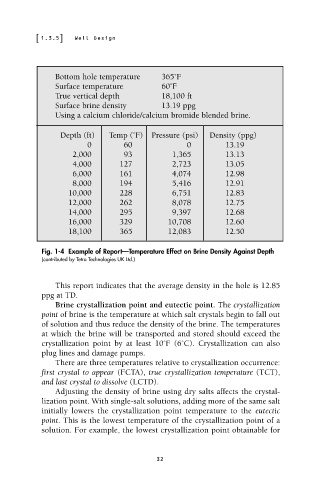
Bottom hole temperature 365˚F
Surface temperature 60˚F
True vertical depth 18,100 ft
Surface brine density 13.19 ppg
Using a calcium chloride/calcium bromide blended brine.
Depth (ft) Temp (˚F) Pressure (psi) Density (ppg)
0 60 0 13.19
2,000 93 1,365 13.13
4,000 127 2,723 13.05
6,000 161 4,074 12.98
8,000 194 5,416 12.91
10,000 228 6,751 12.83
12,000 262 8,078 12.75
14,000 295 9,397 12.68
16,000 329 10,708 12.60
18,100 365 12,083 12.50
Fig. 1-4 Example of Report—Temperature Effect on Brine Density Against Depth
(contributed by Tetra Technologies UK Ltd.)
This report indicates that the average density in the hole is 12.85
ppg at TD.
Brine crystallization point and eutectic point. The crystallization
point of brine is the temperature at which salt crystals begin to fall out
of solution and thus reduce the density of the brine. The temperatures
at which the brine will be transported and stored should exceed the
crystallization point by at least 10˚F (6˚C). Crystallization can also
plug lines and damage pumps.
There are three temperatures relative to crystallization occurrence:
first crystal to appear (FCTA), true crystallization temperature (TCT),
and last crystal to dissolve (LCTD).
Adjusting the density of brine using dry salts affects the crystal-
lization point. With single-salt solutions, adding more of the same salt
initially lowers the crystallization point temperature to the eutectic
point. This is the lowest temperature of the crystallization point of a
solution. For example, the lowest crystallization point obtainable for
32