Page 224 - Pressure Swing Adsorption
P. 224
i .:1:
200 PRESSURE SWING ADSORPTION DYNAMIC MODELING OF A PSA SYSTEM 201
60,------------~--~ recovery, and changing the mtrogen equilibrium affects both purity and
recovery. An 1rnoroved moiecular sieve for mtrogen from air· by pressure
X swmg adsorption would therefore reamre stronger oxygen equilibrium and/or
slower nitrogen diffusion. It should be recalled here that there are limits up
to which such improvements may be effectively ex:pioJted in this type of cycle
I (see Section 3.4).
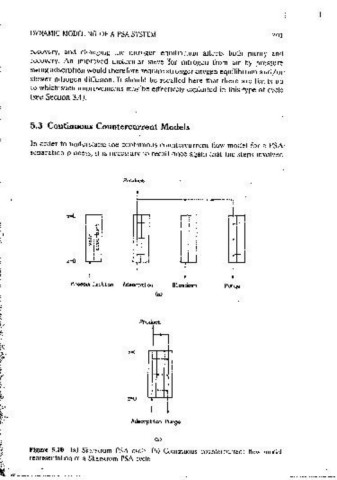
5_3 Continuous Countercurrent Models
Parameters
F\J 12-8 atm) -- In order to understand the contmuous countercurrent ;flow model for a PSA
• l/V 120-35) _,,_ separation process, it 1s necessary to recall once agam that the steos involved
G 10-11 ---·
OPE □
SE (0.5-2) o, •
SK (31)-68) b., •
Pr-od!.rct
0 6 10 16
Mole % oxygen in product
Figure 5.9 Effects of some important operatmg parameters on purity and recovery of
nitrogen m a kinetically controlled PSA air separation process. Adsorptlon/desorp-
t1on time= 60 s, pressunzat1on/blowdown time= 15 s, kinetic and eauilibrium pa-
rameters are given m Table 5.8. The solid line shows the effect of increasmg the
adsorotmn pressure (in the direction of the arrow) for a Skarstrom cycle with no
purge. For two different operatmg pressures the effect of introducing a double-ended
pressure equalization step, as in the modified cycle 1s shown by dotted lines leading to
the pomts ( □). The effect of increasmg purge/feed is shown by cham dotted iine z•O
leading to pomt (0) at purge/feed= 1.0, and the effect of changing the L/ v H ratm
0
from 20 s at nomt ( X) to 35 s at pomt ( +) is mdicated by dot-dash line. The effects
Praeeurizction Adeorption Slowdown
of increasmg and decreasmg the kinetic and equiJibnum oarameters for oxygen and Pwgo
mtrogen (by factors of 1.5 and 2.0, respectively, relative to the exoenmental values) (a)
are also shown. The open symbols show the effect of changing the vaiues for oxygen,
and the closed symbols show the effect of changing the values for mtrogen. The
directions of the mcreasmg vanables ( L / v H, PH, and G) and the kinetic and
0
equilibrmm seiectivities (SK and SE) are indicated by arrows. Product
h
z•L
sure is low. The product purity may be significantly improved m a low-feed ,. -
pressure operation by regenerating the adsorbent bed with product ourge.
However, the recovery 1s reduced by mtroducing purge. Another way of ~-
increasing the product purity when the feed pressure ts low is to increase the ~-
L/vm ratio, but product purge appears to be more efficient. Of course,
1
when operated at high feed pressure a very high-purity product may be z-0
achieved without resorting to any external purge and conseQUentiy, at compa-
rable purity, the recovery is much higher than that from a Skarstrom cycle. """°'1'ttcn Pwgo
The effects of varying the kinetic select1v1ty (SK - D /DN,) and the equilib-
0
rium selectivity (S ! = K 01 /KN) about their experimental values are also (b)
1
illustrated. lt is evident that varying the oxygen diffus1v1ty or equilibnum
Figure 5.10 (a) Skarstrom PSA cvdc. (h) Continuous countcrcurrenl flow model
mainiy affects the ounty. Varying the nitrogen diffusivity affects mainly the reoresentat1on of a Skarstrom PSA c..yclc.