Page 161 - Primer on Enhanced Oil Recovery
P. 161
Chemical EOR 151
the interfacial tension by two or three orders of magnitude. As shown by experi-
mental studies with an increase in the content of organic acids in oil, the interfacial
tension decreases significantly and can be as high as 0.001 mN/m.
The surfactants also increase the wettability of the rock by water. They lead to
emulsification of the residual oil. All this results in much more efficient oil dis-
placement from the surface of the rock. Overall, the processes leads to a significant
decrease in residual oil saturation and further increase in the oil recovery.
A great influence on the properties of the resulting water-oil emulsion is pro-
duced by the organic acids in the oil. The reactions are fairly complex but the over-
all picture can be produced and simplified by the introduction of an activity of the
reservoir oil (as measured by the oil acidity). The acidity is measured by the
amount of potassium hydrophyte to neutralize oil acids. All oils then can be,
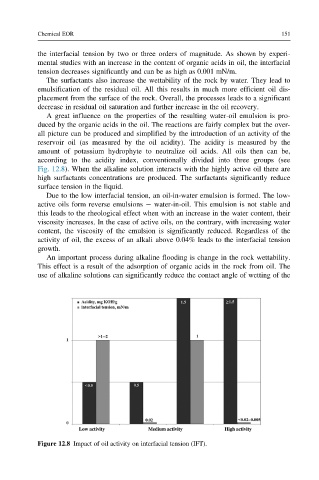
according to the acidity index, conventionally divided into three groups (see
Fig. 12.8). When the alkaline solution interacts with the highly active oil there are
high surfactants concentrations are produced. The surfactants significantly reduce
surface tension in the liquid.
Due to the low interfacial tension, an oil-in-water emulsion is formed. The low-
active oils form reverse emulsions water-in-oil. This emulsion is not stable and
this leads to the rheological effect when with an increase in the water content, their
viscosity increases. In the case of active oils, on the contrary, with increasing water
content, the viscosity of the emulsion is significantly reduced. Regardless of the
activity of oil, the excess of an alkali above 0.04% leads to the interfacial tension
growth.
An important process during alkaline flooding is change in the rock wettability.
This effect is a result of the adsorption of organic acids in the rock from oil. The
use of alkaline solutions can significantly reduce the contact angle of wetting of the
Figure 12.8 Impact of oil activity on interfacial tension (IFT).