Page 167 - Primer on Enhanced Oil Recovery
P. 167
Chemical EOR 157
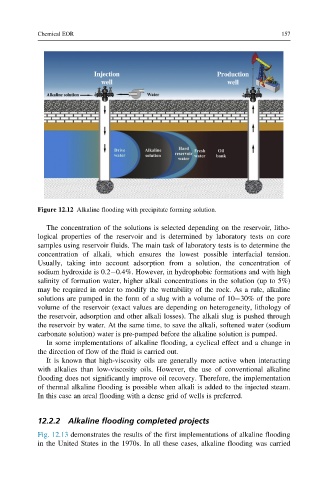
Figure 12.12 Alkaline flooding with precipitate forming solution.
The concentration of the solutions is selected depending on the reservoir, litho-
logical properties of the reservoir and is determined by laboratory tests on core
samples using reservoir fluids. The main task of laboratory tests is to determine the
concentration of alkali, which ensures the lowest possible interfacial tension.
Usually, taking into account adsorption from a solution, the concentration of
sodium hydroxide is 0.2 0.4%. However, in hydrophobic formations and with high
salinity of formation water, higher alkali concentrations in the solution (up to 5%)
may be required in order to modify the wettability of the rock. As a rule, alkaline
solutions are pumped in the form of a slug with a volume of 10 30% of the pore
volume of the reservoir (exact values are depending on heterogeneity, lithology of
the reservoir, adsorption and other alkali losses). The alkali slug is pushed through
the reservoir by water. At the same time, to save the alkali, softened water (sodium
carbonate solution) water is pre-pumped before the alkaline solution is pumped.
In some implementations of alkaline flooding, a cyclical effect and a change in
the direction of flow of the fluid is carried out.
It is known that high-viscosity oils are generally more active when interacting
with alkalies than low-viscosity oils. However, the use of conventional alkaline
flooding does not significantly improve oil recovery. Therefore, the implementation
of thermal alkaline flooding is possible when alkali is added to the injected steam.
In this case an areal flooding with a dense grid of wells is preferred.
12.2.2 Alkaline flooding completed projects
Fig. 12.13 demonstrates the results of the first implementations of alkaline flooding
in the United States in the 1970s. In all these cases, alkaline flooding was carried