Page 182 - Principles of Catalyst Development
P. 182
170 CHAPTER 7
TABLE 7.13. Improved Fluorescen! Indicators"
Wdvelength (/-Lm)
Indicator Acid Neutral pK"
5,7-Dimethyl-I-2-benzacridine .. X() .. 00 +5.5
6, 9-Dichloro-2-met ho xyacrid i ne 5~0 .... 0 +3.0
Dimethyl POPOP 470 420 +0.5
POPOP 460 410 -1.8
" Reference 249.
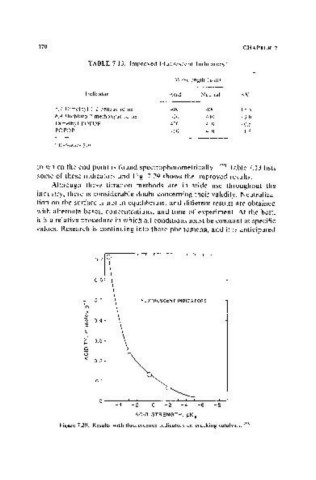
in which the end point is found spectrophotometrically.(249) Table 7_13 lists
some of these indicators and Fig. 7.29 shows the improved results.
Although these titration methods are in wide use throughout the
industry, there is considerable doubt concerning their validity. Neutraliza-
tion on the surface is not in equilibrium, and different results are obtained
with alternate bases, concentrations, and time of experiment. At the best,
it is a relative procedure in which all conditions must be constant at specific
values. Research is continuing into these phenomena, and it is anticipated
0.7
0.6
, 0.5 FLUORESCENT INDICATORS
Cl
'"
QJ
'0 0.4
E
E
;.:
I- 0.3
i5
0
«
0.2
ACID STRENGTH. pKa
Figure 7.29. Results with fluorescence indicators on cracking catalysts. (249 )