Page 183 - Principles of Catalyst Development
P. 183
CAT ALYST CHARACTERIZATION 171
0.05
-
I
CI 0.04
:I: '"
z 0.03
<II
CD
0
E 0.02
E
0.01
200 300 400
TEMPERATURE, ·C
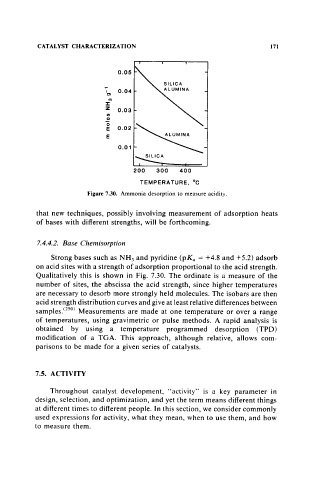
Figure 7.30. Ammonia desorption to measure acidity.
that new techniques, possibly involving measurement of adsorption heats
of bases with different strengths, will be forthcoming.
7.4.4.2. Base Chemisorption
Strong bases such as NH3 and pyridine (pKa = +4.8 and +5.2) adsorb
on acid sites with a strength of adsorption proportional to Ithe acid strength.
Qualitatively this is shown in Fig. 7.30. The ordinate is a measure of the
number of sites, the abscissa the acid strength, since higher temperatures
are necessary to desorb more strongly held molecules. The isobars are then
acid strength distribution curves and give at least relative differences between
samples.(250) Measurements are made at one temperature or over a range
of temperatures, using gravimetric or pulse methods. A rapid analysis is
obtained by using a temperature programmed desorption (TPD)
modification of a TGA. This approach, although relative, allows com-
parisons to be made for a given series of catalysts.
7.5. ACTIVITY
Throughout catalyst development, "activity" is a key parameter in
design, selection, and optimization, and yet the term means different things
at different times to different people. In this section, we consider commonly
used expressions for activity, what they mean, when to use them, and how
to measure them.