Page 229 - Principles of Catalyst Development
P. 229
218 CHAPTER 8
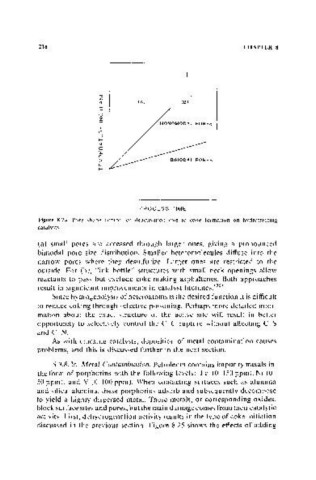
,--I L,
, I
w L~
(fJ
« (8)
w
a:
u
z
w MONO MODAL PORES
a:
::J
....
«
a:
w
a..
:::;
w
....
L ___ _
PROCESS TIME
Figure 8.24. Pore shape control of deactivation due to coke formation on hydrotreating
catalysts.
(a) small pores are accessed through larger ones, gIVlTlg a pronounced
bimodal pore size distribution. Smaller heteromolecules diffuse into the
narrow pores where they desulfurize. Larger ones are restricted to the
outside. For (b), "ink bottle" structures with small neck openings allow
reactants to pass but exclude coke-making asphaltenes. Both approaches
result in signit1cant improvements in catalyst lifetimes.(36)
Since hydrogenolysis of heteroatoms is the desired function it is difficult
to reduce coking through selective poisoning. Perhaps more detailed infor-
mation about the exact structure of the active site will result in better
opportunity to selectively control the C-C rupture without affecting C-S
and C-N.
As with cracking catalysts, deposition of metal contamination causes
problems, and this is discussed further in the next section.
8.3.8.2c. Metal Contamination. Petroleum contains impurity metals in
the form of porphorins with the following levels: Fe (0-150 ppm), Ni (0-
50 ppm), and V (0-100 ppm). When contacting surfaces such as alumina
and silica-alumina, these porphorins adsorb and subsequently decompose
to yield a highly dispersed metal. These metals, or corresponding oxides,
block surface sites and pores, but the main damage comes from their catalytic
activity. First, dehydrogenation activity results in the type of coke initiation
discussed in the previous section. Figure 8.25 shows the etIects of adding