Page 44 - Principles of Catalyst Development
P. 44
30 CHAPTER 2
10 7
10 6 d=do(l+at m )
E
c: - a = a ( M P, Temp)
c:: 10 5
w
I-
W
::E 10 4
::!
0
w 10 3
I-
..J
..J
<t 10 2
l-
V)
>-
c::
u 10
IT
1000 1500 2000
MELTING POINT, ·C
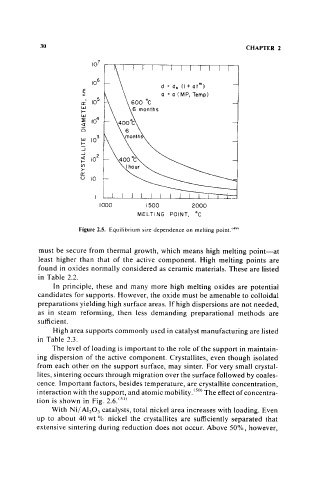
Figure 2.5. Equilibrium size dependence on melting point. (49)
must be secure from thermal growth, which means high melting point-at
least higher than that of the active component. High melting points are
found in oxides normally considered as ceramic materials. These are listed
in Table 2.2.
In principle, these and many more high melting oxides are potential
candidates for supports. However, the oxide must be amenable to colloidal
preparations yielding high surface areas. If high dispersions are not needed,
as in steam reforming, then less demanding preparational methods are
sufficient.
High area supports commonly used in catalyst manufacturing are listed
in Table 2.3.
The level of loading is important to the role of the support in maintain-
ing dispersion of the active component. Crystallites, even though isolated
from each other on the support surface, may sinter. For very small crystal-
lites, sintering occurs through migration over the surface followed by coales-
cence. Important factors, besides temperature, are crystallite concentration,
interaction with the support, and atomic mobility. (50) The effect of concentra-
tion is shown in Fig. 2.6. (51)
With Nil Al 20 3 catalysts, total nickel area increases with loading. Even
up to about 40 wt % nickel the crystallites are sufficiently separated that
extensive sintering during reduction does not occur. Above 50%, however,