Page 69 - Principles of Catalyst Development
P. 69
56 CHAPTER 4
atoms, such as copper, merely dilute the surface, so that the number of
ensembles decreases in a manner consistent with probability statistics. Thus
in Fig. 4.6, nickel retains its electronic character and activity drops as the
number of adjacent nickel atoms decreases with copper addition.
Historically, as the geometric and electronic theories began to fade, a
new model emerged, incorporating features of both. A localized bond theory,
the model is based on concepts in the ligand field treatment of bonding in
inorganic and organometallic complexes. Face-centered cubic nickel has
octahedral symmetry with its neighbors, whose electric field splits the
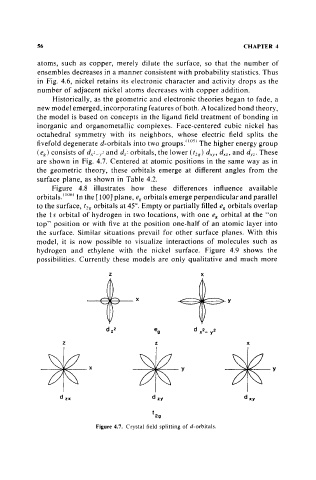
fivefold degenerate d-orbitals into two groups.iI05) The higher energy group
(e g ) consists of dx 2 _ y ' and dz 2 orbitals, the lower (t 1g ) dxy , dxz , and d),z' These
are shown in Fig. 4.7. Centered at atomic positions in the same way as in
the geometric theory, these orbitals emerge at different angles from the
surface plane, as shown in Table 4.2.
Figure 4.8 illustrates how these differences influence available
orbitals. (106) In the [100J plane, e g orbitals emerge perpendicular and parallel
to the surface, t 2g orbitals at 45°. Empty or partially filled eg orbitals overlap
the 1 s orbital of hydrogen in two locations, with one eg orbital at the "on
top" position or with five at the position one-half of an atomic layer into
the surface. Similar situations prevail for other surface planes. With this
model, it is now possible to visualize interactions of molecules such as
hydrogen and ethylene with the nickel surface. Figure 4.9 shows the
possibilities. Currently these models are only qualitative and much more
z x
--+--II,...-}-- x
z z x
---,*E--_X ---3+E--- Y Y
dzx d zy d xy
t 2g
Figure 4.7. Crystal field splitting of d-orbitals.