Page 108 - Principles of Catalyst Development
P. 108
96 CHAPTER 6
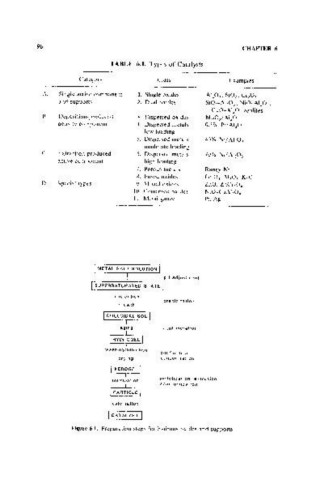
TABLE 6.1. Types of Catalysts
Category Class Examples
A. Single active component 1. Single oxides AI,OJ, SiO" Cr,O,
and supports 2, Dual oxides SiOrAI,O" NiO-AIPJ'
CuO-AI,O, zeolites
B, Deposition-produced 3, Dispersed oxides MoO,/ AI,O,
activity component 4, Dispersed metals 0,3% Pt/ Al,Ol
low loading
5, Dispersed metals 40% Nil AI,O,
moderate loading
C. Extraction-produced 6, Dispersed metals 70% Nil AI,O,
active component high loading
7, Porous metals Raney Ni
g, Fused oxides Fe,04-AI,O,-K,O
D, Special types <), Mixed oxides ZnO, ZnCr,04
10, Cemented oxides NiO-CaAI,04
I 1. Metal gauLe Pt,Ag
_._- ----_ ..
I METAL SAL T SOLUTION I
! pH adjustment
nucleation
I precipitation
growth
aging agglomeration
I HYDR:OGEL I
washing/tiltra tion
I purification
drying concentration
I XERtGEL I
pelletization, extrusion,
formulation sph eroidiz a t i on
, PAR~ICLE I
calcination
I
'CATALYST I
Figure 6.1. Preparation steps for hydrous oxides and supports,