Page 110 - Principles of Catalyst Development
P. 110
98 CHAPTER 6
PRECIPITATION /
/
/
z /
o
I- /
< SUPERSATURATED / .D
a: REGION / .,11
I-
z \ ,/ B~-r--... A ••••••
UJ
() /
Z ,,/ ,,'<.. .. ,.,/""
o
,.. ., ...... .
(J ,,/ ,.
..
.' ' SOLUTION
SOLUBILITy ••• ••••
CURVE ••••
......
TEMPERATURE
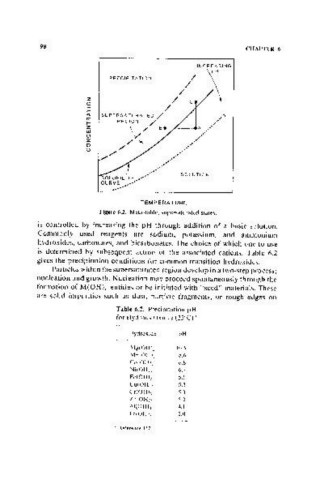
Figure 6.2. Metastable, supersaturated states.
is controlled by increasing the pH through addition of a basic solution.
Commonly used reagents are sodium, potassium, and ammonium
hydroxides, carbonates, and bicarbonates. The choice of which one to use
is determined by subsequent action of the associated cations. Table 6.2
gives the precipitation conditions for common transition hydroxides.
Particles within the supersaturated region develop in a two-step process;
nucleation and growth. Nucleation may proceed spontaneously through the
formation of M(OH)" entities or be initiated with "seed" materials. These
are solid impurities such as dust, particle fragments, or rough edges on
Table 6.2. Precipitation pH
for Hydrous Oxides (25°(')"
Hydroxide pH
Mg(OH), 10.5
Mn(OH), 8.6
Co(OH), 6.8
Ni(OH), 6.7
Fe(OH), 5.5
Cu(OH), 5.3
Cr(OH), 5.3
Zn(OH), 5.2
AI(OH), 4.1
Fe(OH), 2.0
------
u Reference 1,\7.