Page 230 - Principles of Catalyst Development
P. 230
CATALYST DEACTIVATION 219
50
W
SiO]-AI 20 3 !':
45 ...J
0
(/)
«
(!)
40 I-
Z
W
U
w
35 a::
a.
w
::;
30 :::l
...J
z 0
>
o
~ 6 f--------------l 25
«
u
I-
~ 5
u
a::
w
a. 4
l-
I
(!)
~ 30~~==~~L-----L--~
> 0.5 1.0
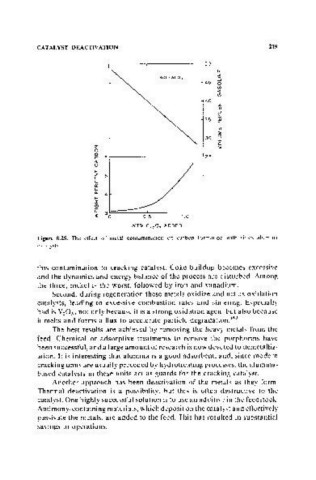
Figure 8.25. The effect of metal contamination on carbon formation with silica alumina
catalysts.
this contamination to cracking catalyst. Coke buildup becomes excessive
and the dynamics and energy balance of the process are disturbed. Among
the three, nickel is the worst, followed by iron and vanadium.
Second, during regeneration these metals oxidize and act as oxidation
catalysts, leading to excessive combustion rates and sintering. Especially
bad is V 20" not only because it is a strong oxidation agent but also because
it melts and forms a flux to accelerate particle degradation. (69)
The best results are achieved by removing the heavy metals from the
feed. Chemical or adsorptive treatments to remove the porphorins have
been successful, and a large amount of research is now devoted to demetalliz-
ation. It is interesting that alumina is a good adsorbent, and, since modern
cracking units are usually preceded by hydrotreating processes, the alumina-
based catalysts in these units act as guards for the cracking catalyst.
Another approach has been deactivation of the metals as they form.
Thermal deactivation is a possibility, but this is often destructive to the
catalyst. One highly successful solution is to use an additive in the feedstock.
Antimony-containing materials, which deposit on the catalyst and effectively
passivate the metals, are added to the feed. This has resulted in substantial
savings in operations.