Page 228 - Principles of Catalyst Development
P. 228
CATALYST DEACTIVATION 217
CATALYTIC REFORMING
>-
r- YEARS
>
r-
()
«
PROCESS TIME
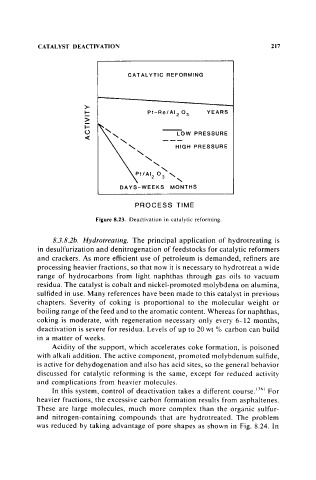
Figure 8.23. Deactivation in catalytic reforming.
S.3.S.2b. Hydrotreating. The principal application of hydrotreating is
in desulfurization and denitrogenation of feedstocks for catalytic reformers
and crackers. As more efficient use of petroleum is demanded, refiners are
processing heavier fractions, so that now it is necessary to hydrotreat a wide
range of hydrocarbons from light naphthas through gas oils to vacuum
residua. The catalyst is cobalt and nickel-promoted molybdena on alumina,
sulfided in use. Many references have been made to this catalyst in previous
chapters. Severity of coking is proportional to the molecular weight or
boiling range of the feed and to the aromatic content. Whereas for naphthas,
coking is moderate, with regeneration necessary only every 6-12 months,
deactivation is severe for residua. Levels of up to 20 wt % carbon can build
in a matter of weeks.
Acidity of the support, which accelerates coke formation, is poisoned
with alkali addition. The active component, promoted molybdenum sulfide,
is active for dehydogenation and also has acid sites, so the general behavior
discussed for catalytic reforming is the same, except for reduced activity
and complications from heavier molecules.
In this system, control of deactivation takes a different course. Dol For
heavier fractions, the excessive carbon formation results from asphaltenes.
These are large molecules, much more complex than the organic sulfur-
and nitrogen-containing compounds that are hydrotreated. The problem
was reduced by taking advantage of pore shapes as shown in Fig. 8.24. In