Page 224 - Principles of Catalyst Development
P. 224
CATALYST DEACTIVATION 213
a'!
lO
f-
~
a"
w
w
u.
z
0 5
z
0
(D
a:
«
<..l
OL-__ ~ ____ ~ __ ~ ____ ~ ____ ~ __ -J
40 50 60 70
CONVERSION, VOL %
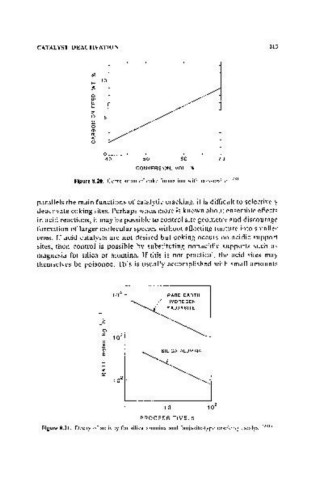
Figure 8,20. Correlation of coke formation with conversion f2R1 )
parallels the main functions of catalytic cracking, it is difficult to selectively
deactivate coking sites. Perhaps when more is known about ensemble effects
in acid reactions, it may be possible to control site geometry and discourage
formation of larger molecular species without affecting rupture into smaller
ones, If acid catalysts are not desired but coking occurs on acidic support
sites, then control is possible by substituting nonacidic supports such as
magnesia for silica or alumina. If this is not practical, the acid sites may
themselves be poisoned. This is usually accomplished with small amounts
/ RARE EARTH-
/ HYDROGEN
FAUJASITE
',-
.c
(0)
""
I/)
Q)
'0
E
UJ
f--
c(
a:
10
PROCESS TIME. s
Figure 8.21. Decay of activity for silica alumina and faujasite-type cncking catalyst f2XII