Page 222 - Principles of Catalyst Development
P. 222
CATALYST DEACTIVATION 211
Within this framework, coking phenomena are best considered in terms
of three common operations: (1) acid coking, for example, cracking on
catalysts; (2) dehydrogenation coking, as in catalytic reforming; and (3)
dissociative coking, found in steam reforming.
8.3.8.1. Acid Coking
Acid coke forms on silica alumina and zeolite cracking catalysts and
on acidic supports. Coke forming tendency is directly related to acidity.
There are two major types of carbon structures existing as highly dispersed
phases in the pores. Most of the coke is present as pseudographitic or
turbostratic, random-layer lattices, similar to graphite, with a composition
of CH OA to CH 05 ' The remainder consists of poorly organized polynuclear
aromatic macromolecules. (279)
There is strong evidence that acid coke originates with aromatic and
olefinic hydrocarbons, either initially in the feed or generated as intermedi-
ates during the cracking process. Coke buildup correlates well with aro-
matic/naphthene ratios in gas oils and also with the basicity of polynuclear
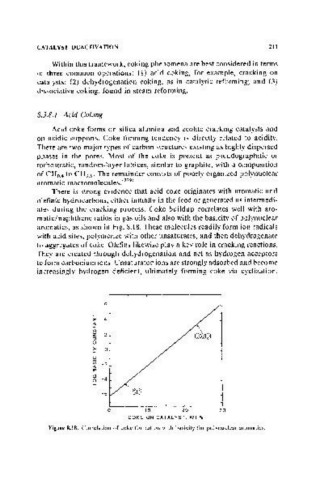
aromatics, as shown in Fig. 8.18. These molecules readily form ion radicals
with acid sites, polymerize with other unsaturates, and then dehydrogenate
to aggregates of coke. Olefins likewise playa key role in cracking reactions.
They are created through dehydrogenation and act as hydrogen acceptors
to form carbonium ions. Unsaturated ions are strongly adsorbed and become
increasingly hydrogen deficient, ultimately forming cok,~ via cyclization.
6
f-
Z 4
4:
f-
(/)
z 2
0
a
>- 0
f-
a
(/) -2
4:
CD
(!l
0 -4
...J
-6
0 10 20 30
COKE ON CATAL YST. WT %
Figure S.lS. Correlation of coke formation with basicity for polynuclear aromatics.