Page 223 - Principles of Catalyst Development
P. 223
212 CHAPTER 8
Increased acid site strength and density favor coke formation, which is a
fact of life in catalytic cracking and must either be accepted or the catalyst
modified to hinder formation or to facilitate removal.
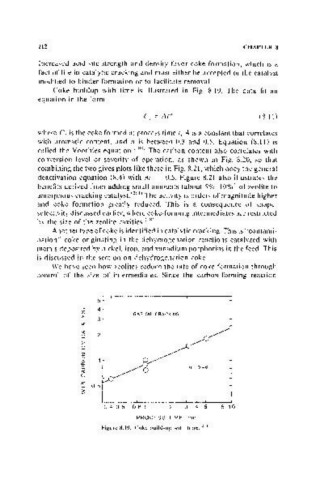
Coke buildup with time is illustrated in Fig. 8.19. The data fit an
equation in the form
(8.11)
where C is the coke formed at process time t, A is a constant that correlates
with aromatic content, and n is between 0.3 and 0.5. Equation (8.1l) is
called the Voorhies equation. (280) The carbon content also correlates with
conversion level or severity of operation, as shown in Fig. 8.20, so that
combining the two gives plots like these in Fig. 8.21, which obey the general
deactivation equation (8.4) with m = -0.5. Figure 8.21 also illustrates the
benefits derived from adding small amounts (about 5 'Yo -1 0%) of zeolite to
amorphous cracking catalyst. (26)) The activity is orders of magnitude higher
and coke formation greatly reduced. This is a consequence of shape-
selectivity discussed earlier, where coke-forming intermediates are restricted
by the size of the zeolite cavities. (2H) (
Another type of coke is identified in catalytic cracking. This is "contami-
nation" coke originating in the dehydrogenation reactions catalyzed with
metals deposited by nickel, iron, and vanadium porphorins in the feed. This
is discussed in the section on dehydrogenation cokc.
We have secn how zeolites reduce the rate of coke formation through
control of the size of intermediates. Since the carbon-forming reaction
5
l- 4
ff) GAS OIL CRACKING
>-
...J 3
«
I-
«
o 2
z
o
z
o
CD
Il:
«
o
't. 0.5
:s:
L-----'----'----'----L-L-~
0.4 0.5 0.8 1 2 3 4 5 8 10
PROCESS TIME, min
Figure 8.19. Coke build-up with time.' '.'1,