Page 42 - Principles of Catalyst Development
P. 42
28 CHAPTER 2
invoked to explain and predict differences in, for example, metals or crystal
planes.
Semiconducting oxides and sulfides constitute a large class of catalytic
materials. '45 ) Electron donor and acceptor levels provide redox-type activa-
tion, but surface configurations are more complicated than with metals.
Greater geometric complexity leads to more selective redox reactions, such
as partial oxidation, hydrodesulfurization, and denitrogenation. Insulators
do not readily promote charge transfer, but surface sites with localized
protons are favored. (46) Acid-like in nature, these sites promote carbonium
ion mechanisms, resulting in typically acid-catalyzed reactions such as
isomerization or cracking.
With these brief introductory remarks, the reader is referred to Chapter
4 for more explicit descriptions of active components.
2.3.2. Support
Supports, or carriers, perform many functions, but most important is
maintenance of high surface area for the active component. This is best
illustrated with platinum, an important catalytic metal widely used for
catalytic reforming(1 I) and automobile exhaust clean-up. (47) For high
activity, platinum crystallites must have the highest surface area possible.
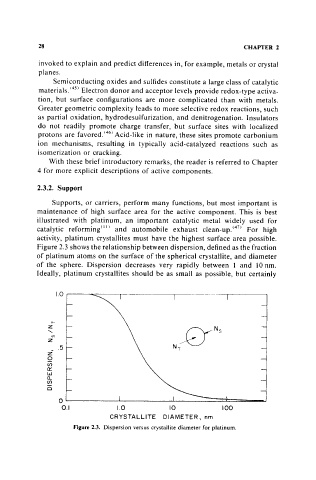
Figure 2.3 shows the relationship between dispersion, defined as the fraction
of platinum atoms on the surface of the spherical crystallite, and diameter
of the sphere. Dispersion decreases very rapidly between 1 and 10 nm.
Ideally, platinum crystallites should be as small as possible, but certainly
1.0 ,..----=:----r--------r--------,.----...,
...
z
......
z (/)
.5
z
Q
(f)
Il:
W
11.
(f)
0
0
0.1 1.0 10 100
CRYSTALLITE DIAMETER, nm
Figure 2.3. Dispersion versus crystallite diameter for platinum.