Page 43 - Principles of Catalyst Development
P. 43
STRUCTURE OF CATALYSTS 29
in the 0.5-5 nm range. Platinum spheres of this size can be made as colloidal
platinum black. (48) Attempts to use the colloid as a catalyst at reaction
temperatures rapidly leads to sintering or agglomeration of the crystallites
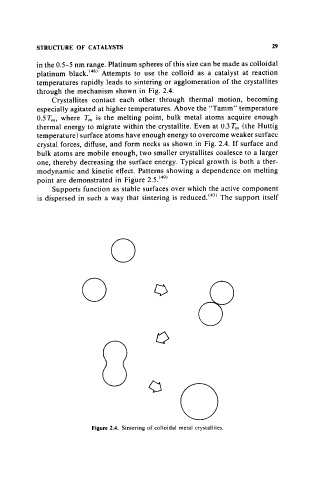
through the mechanism shown in Fig. 2.4.
Crystallites contact each other through thermal motion, becoming
especially agitated at higher temperatures. Above the "Tamm" temperature
O.5Tm, where Tm is the melting point, bulk metal atoms acquire enough
thermal energy to migrate within the crystallite. Even at 0.3 Tm (the Huttig
temperature) surface atoms have enough energy to overcome weaker surface
crystal forces, diffuse, and form necks as shown in Fig. 2.4. If surface and
bulk atoms are mobile enough, two smaller crystallites coalesce to a larger
one, thereby decreasing the surface energy. Typical growth is both a ther-
modynamic and kinetic effect. Patterns showing a dependence on melting
point are demonstrated in Figure 2.5.(49)
Supports function as stable surfaces over which the active component
is dispersed in such a way that sintering is reduced. (43) The support itself
o
o o
o
o
Figure 2.4. Sintering of colloidal metal crystallites.