Page 66 - Principles of Catalyst Development
P. 66
CATALYTIC MATERIALS 53
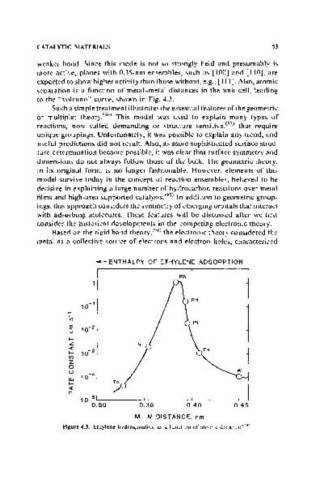
weaker bond. Since this mode is not so strongly held and presumably is
more active, planes with 0.35-nm ensembles, such as [100] and [ItO], are
expected to show higher activity than those without, e.g., [111]. Also, atomic
separation is a function of metal-metal distances in the unit cell, leading
to the "volcano" curve, shown in Fig. 4.3.
Such a simple treatment illustrates the essential features of the geometric
or multiplet theory.(96) This model was used to explain many types of
reactions, now called demanding or structure sensitive, (97) that require
unique groupings. Unfortunately, it was possible to explain any trend, and
useful predictions did not result. Also, as more sophisticated surface struc-
ture determination became possible, it was clear that surface symmetry and
dimensions do not always follow those of the bulk. The geometric theory,
in its original form, is no longer fashionable. However, elements of this
model survive today in the concept of reaction ensembles, believed to be
decisive in explaining a large number of hydrocarbon reactions over metal
films and high-area supported catalysts.(95) In addition to geometric group-
ings, this approach considers the symmetry of emerging orbitals that interact
with adsorbing molecules. These features will be discussed after we first
consider the historical developments in the competing electronic theory.
Based on the rigid band theory,(98) the electronic theory considered the
metal as a collective source of electrons and electron holes, characterized
- ENTHALPY OF ETHYLENE ADSORPTION
Rh
10- 1
~
I
~
E 10- 2
u
I-
Z
<1:
l- 10- 3
(/)
Z
0
()
W 10- 4
l-
e(
ex:
10- 5
0.30 0.35 0.40 0.45
M - M DISTANCE, nm
Figure 4.3. Ethylene hydrogenation as a function of atomic distance. 124 )