Page 73 - Principles of Catalyst Development
P. 73
60 CHAPTER 4
.8
: CORNER
I ~(111] FACE
I
C/) •7 · I
w I I
I- I I
en .6 I
w
()
~ .5
a:
:::>
C/) .4
u.
o
z
o
~
()
« (100] FACE
a:
u.
(111]-(100] EDGE
o 2 3 4 5 6 7 8 9 10
RADIUS, nm
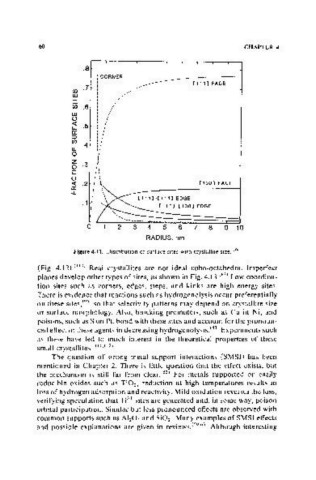
Figure 4.11. Distribution of surface sites with crystallite size. II08 )
(Fig. 4.12). (I 101 Real crystallites are not ideal cubo-octahedra. Imperfect
planes develop other types of sites, as shown in Fig. 4.13.(83) Low coordina-
tion sites such as corners, edges, steps, and kinks are high energy sites.
There is evidence that reactions such as hydrogenolysis occur preferentially
on these sites,19S 1 so that selectivity patterns may depend on crystallite size
or surface morphology. Also, blocking promoters, such as Cu in Ni, and
poisons, such as S on Pt, bond with these sites and account for the pronoun-
ced effect of these agents in decreasing hydrogenolysis.(831 Experiments such
as these have led to much interest in the theoretical properties of these
small crystallites.( 111,IIZ)
The question of strong metal support interactions (SMSI) has been
mentioned in Chapter 2. There is little question that the effect exists, but
the mechanism is still far from clear.(63) For metals supported on easily
reducible oxides such as Ti0 2 , reduction at high temperatures results in
loss of hydrogen adsorption and reactivity. Mild oxidation reverses the loss,
verifying speculation that Ti3+ sites are generated and, in some way, poison
orbital participation. Similar but less pronounced effects are observed with
common supports such as Alz0 3 and Si0 2 . Many examples of SMSI effects
and possible explanations are given in reviews.(59,63) Although interesting