Page 72 - Principles of Catalyst Development
P. 72
CATALYTIC MATERIALS 59
The model is consistent with observed patterns in adsorption and
reactivity across the periodic table. Both spacing and orbital occupancy are
involved, so that combinations of ensemble and ligand effects are necessary
to explain dependencies.
Finally, alloying patterns are justified. Figure 4.6 shows cyclopropane
hydrogenation decreasing uniformly with surface eu concentration. Each
nickel atom retains its orbital integrity, and the effect of copper is simply
to dilute. For instance, if n surface nickel sites are required to generate an
absorbed species, then a surface copper fraction feu results in an activity
decrease, AI A o, given by
A. )n
Ao = (1 - feu (4.1 )
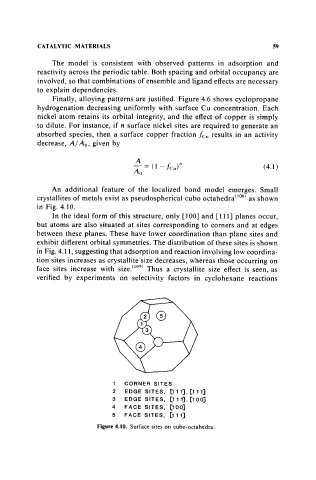
An additional feature of the localized bond model emerges. Small
crystallites of metals exist as pseudospherical cubo octahedra(108) as shown
in Fig. 4.10.
In the ideal form of this structure, only [100] and [111] planes occur,
but atoms are also situated at sites corresponding to corners and at edges
between these planes. These have lower coordination than plane sites and
exhibit different orbital symmetries. The distribution of these sites is shown
in Fig. 4.11, suggesting that adsorption and reaction involving low coordina-
tion sites increases as crystallite size decreases, whereas those occurring on
face sites increase with sizeyo9) Thus a crystallite size effect is seen, as
verified by experiments on selectivity factors in cyclohexane reactions
1 CORNER SITES
2 EDGE SITES, [111], [111]
3 EDGE SITES, [111], [100]
4 F ACE SIT E S , [1 00]
5 FACE SITES, [111]
Figure 4.10. Surface sites on cubo-octahedra.