Page 124 - Radiochemistry and nuclear chemistry
P. 124
110 Radiochemistry and Nuclear Chemistry
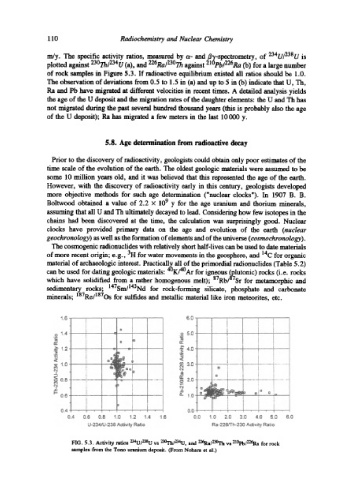
m/y. The specific activity ratios, measured by ix- and BT-spectrometry, of 234U/238U is
plotted against 230Th/234U (a), and 226Ra/23~ against 21~ (b) for a large number
of rock samples in Figure 5.3. If radioactive equilibrium existed all ratios should be 1.0.
The observation of deviations from 0.5 to 1.5 in (a) and up to 5 in (b) indicate that U, Th,
Ra and Pb have migrated at different velocities in recent times. A detailed analysis yields
the age of the U deposit and the migration rates of the daughter elements: the U and Th has
not migrated during the past several hundred thousand years (this is probably also the age
of the U deposit); Ra has migrated a few meters in the last 10000 y.
5.8. Age determination from radioactive decay
Prior to the discovery of radioactivity, geologists could obtain only poor estimates of the
time scale of the evolution of the earth. The oldest geologic materials were assumed to be
some 10 million years old, and it was believed that this represented the age of the earth.
However, with the discovery of radioactivity early in this century, geologists developed
more objective methods for such age determination ("nuclear clocks"). In 1907 B. B.
Boltwood obtained a value of 2.2 x 109 y for the age uranium and thorium minerals,
assuming that all U and Th ultimately decayed to lead. Considering how few isotopes in the
chains had been discovered at the time, the calculation was surprisingly good. Nuclear
clocks have provided primary data on the age and evolution of the earth (nuclear
geochronology) as well as the formation of elements and of the universe (cosmochronology).
The cosmogenic radionuclides with relatively short half-lives can be used to date materials
of more recent origin; e.g., 3H for water movements in the geosphere, and 14C for organic
material of archaeologic interest. Practically all of the primordial radionuclides (Table 5.2)
can be used for dating geologic materials: 4~176 for igneous (plutonic) rocks (i.e. rocks
87 ,87
which have solidified from a rather homogenous melt); Rb/ Sr for metamorphic and
sedimentary rocks; 147 Sm/ 143 Nd for rock-forming silicate, phosphate and carbonate
minerals; 187Re/187Os for sulfides and metallic material like iron meteorites, etc.
FIG. 5.3. Activity ratios X~4U/2"~U vs x~/234U, and 2X'Ra/2"~ vs 21~ for rock
samples from the Tono uranium deposit. (From Nohara et al.)