Page 129 - Radiochemistry and nuclear chemistry
P. 129
Radionuclides in Nature 115
,5000
4000
3000
-r
~I: 2000
1000
00 1 2 3 4x 107
4OK/~Ar
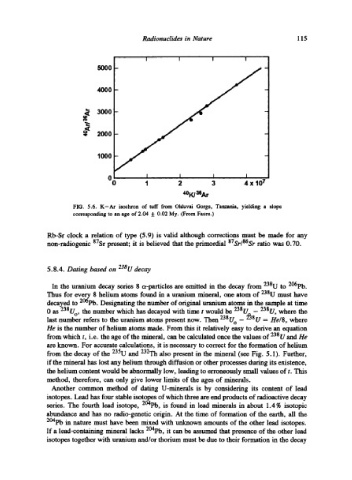
FIG. 5.6. K-Ar isochron of tuff from Olduvai Gorge, Tanzania, yielding a slope
corresponding to an age of 2.04 5:0.02 My. (From Faure.)
Rb-Sr clock a relation of type (5.9) is valid although corrections must be made for any
non-radiogenic 87Sr present; it is believed that the primordial 87Sr/86Sr ratio was 0.70.
5.8.4. Dating based on 2SSU decay
In the uranium decay series 8 a-particles are emitted in the decay from 238U to 206pb.
Thus for eve~ 8 helium atoms found in a uranium mineral, one atom of 238U must have
decayed to 2~T
Pb. Designating the number of original uranium atoms in the sample at time
0 as 238 Uo ' the number which has decayed with time t would be 238 U_ - 238 U, where the
last number refers to the uranium atoms present now. Then 238 U o - ~8 U = He~8, where
He is the number of helium atoms made. From this it relatively easy to derive an equation
from which t, i.e. the age of the mineral, can be calculated once the values of 238U and He
are known. For accurate calculations, it is necessary to correct for the formation of helium
from the decay of the 235U and 232Th also present in the mineral (see Fig. 5.1). Further,
if the mineral has lost any helium through diffusion or other processes during its existence,
the helium content would be abnormally low, leading to erroneously small values of t. This
method, therefore, can only give lower limits of the ages of minerals.
Another common method of dating U-minerals is by considering its content of lead
isotopes. Lead has four stable isotopes of which three are end products of radioactive decay
series. The fourth lead isotope, 204pb, is found in lead minerals in about 1.4 % isotopic
abundance and has no radio-genetic origin. At the time of formation of the earth, all the
204pb in nature must have been mixed with unknown amounts of the other lead isotopes.
If a lead-containing mineral lacks 2~ it can be assumed that presence of the other lead
isotopes together with uranium and/or thorium must be due to their formation in the decay