Page 131 - Radiochemistry and nuclear chemistry
P. 131
Radionuclides in Nature 117
1000
207pb/236 U
100
oo
O 10
< 207pb
n~
o 20Spb/238 u
o
F- 1
< f
,// 208pb/2"32Th
0.1
/
0.01 I1/ I I I I I I I
0 1 2 3 4 5 6 7
AGE (UNITS OF 10 9 YEARS)
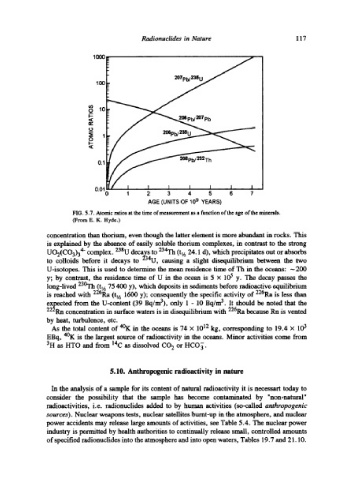
FIG. 5.7. Atomic ratios at the time of measurement as a function of the age of the minerals.
(From E. K. Hyde.)
concentration than thorium, even though the latter element is more abundant in rocks. This
is explained by the absence of easily soluble thorium complexes, in contrast to the strong
UO2(CO3)34- complex. 238U decays to 234Th (t~ h 24.1 d), which precipitates out or absorbs
to colloids before it decays to 234U, causing a slight disequilibrium between the two
U-isotopes. This is used to determine the mean residence time of Th in the oceans: -200
y; by contrast, the residence time of U in the ocean is 5 x 105 y. The decay passes the
long-lived 23~ (tlA 75 400 y), which deposits in sediments before radioactive equilibrium
is reached with 226Ra (tt h 1600 y); consequently the specific activity of 226Ra is less than
expected from the U-content (39 Bq/m3), only 1 - 10 Bq/m 3. It should be noted that the
222Rn concentration in surface waters is in disequilibrium with 226Ra ~use Rn is vented
by heat, turbulence, etc.
As the total content of 4~ in the oceans is 74 x 1012 kg, corresponding to 19.4 • 103
EBq, 4~ is the largest source of radioactivity in the oceans. Minor activities come from
3H as HTO and from 14C as dissolved CO 2 or HCO 3.
5.10. Anthropogenic radioactivity in nature
In the analysis of a sample for its content of natural radioactivity it is necessart today to
consider the possibility that the sample has become contaminated by "non-natural"
radioactivities, i.e. radionuclides added to by human activities (so-called anthropogenic
sources). Nuclear weapons tests, nuclear satellites burnt-up in the atmosphere, and nuclear
power accidents may release large amounts of activities, see Table 5.4. The nuclear power
industry is permitted by health authorities to continually release small, controlled amounts
of specified radionuclides into the atmosphere and into open waters, Tables 19.7 and 21.10.