Page 43 - Reliability and Maintainability of In service Pipelines
P. 43
32 Reliability and Maintainability of In-Service Pipelines
the reinforcing steel are in close proximity to each other, therefore acting as a
direct pathway for the constant continuation of the corrosion mechanism. This
constant cycle results in loss of reinforcing steel material from anodic sites and
creates pitting on the surface. As corrosion progresses the exposed pitted area of
the reinforcing steel becomes less alkaline, which results in further loss of mate-
rial from the bottom of the pitting. This loss of material is a “progressive”
action and reaches a point at which the reinforcing steel is wasted away and no
longer able to withstand applied loads, ultimately resulting in failure (Daily,
2017).
Corrosion Geometry
Uniform Corrosion Uniform corrosion is the most abundant in steel reinforce-
ments, with the entire or large fraction of the surface area of the exposed steel bar
evenly covered with rust, often observed as a reddish color. This reddish color
can also be observed in concrete that is porous and wet, in which corrosion
migrates through the concrete cover, and is commonly presented in the form of
stains on the concrete surface. For dry concretes, however, corrosion can progress
further by deforming the concrete cover and can result in spalling and cracking.
Pitting Corrosion
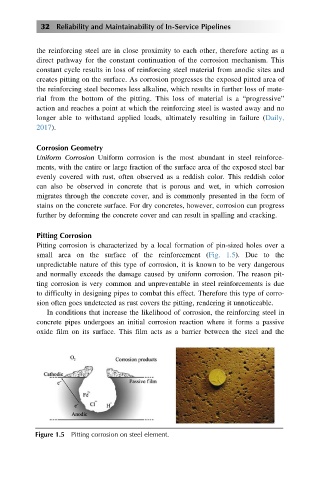
Pitting corrosion is characterized by a local formation of pin-sized holes over a
small area on the surface of the reinforcement (Fig. 1.5). Due to the
unpredictable nature of this type of corrosion, it is known to be very dangerous
and normally exceeds the damage caused by uniform corrosion. The reason pit-
ting corrosion is very common and unpreventable in steel reinforcements is due
to difficulty in designing pipes to combat this effect. Therefore this type of corro-
sion often goes undetected as rust covers the pitting, rendering it unnoticeable.
In conditions that increase the likelihood of corrosion, the reinforcing steel in
concrete pipes undergoes an initial corrosion reaction where it forms a passive
oxide film on its surface. This film acts as a barrier between the steel and the
Figure 1.5 Pitting corrosion on steel element.