Page 368 - Reservoir Formation Damage
P. 368
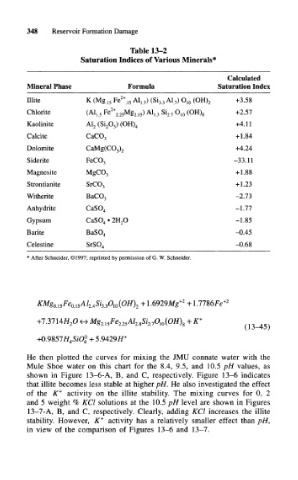
348 Reservoir Formation Damage
Table 13-2
Saturation Indices of Various Minerals*
Calculated
Mineral Phase Formula Saturation Index
Illite K (Mg 15 Fe 2+ 15 Al, 7) (Si 3 3 Al 7) O 10 (OH) 2 +3.58
Chlorite (A1 L5 Fe 2+ 2 25Mg 2, 5) A1 L3 Si 2 7 O 10 (OH) 8 +2.57
Kaolinite A1 2 (Si 20 5) (OH) 4 +4.11
Calcite CaCO 3 +1.84
Dolomite CaMg(C0 3) 2 +4.24
Siderite FeCO 3 -33.11
Magnesite MgC0 3 +1.88
Strontianite SrCO 3 +1.23
Witherite BaCO 3 -2.73
Anhydrite CaSO 4 -1.77
Gypsum CaSO 4 • 2H 2O -1.85
Barite BaSO 4 -0.45
Celestine SrSO 4 -0.68
* After Schneider, ©1997; reprinted by permission of G. W. Schneider.
+ l.6929Mg +2 +
KMg OA5Fe OA5Al 24Si 33O w(OH) 2
+7.3714// 20 ^ Mg 2A5Fe 2. 25A/ 2. 8 S/ 2. 70 10(0//) 8 + K +
(13-45)
+0.9857/f 4S*0 4° +5.9429// +
He then plotted the curves for mixing the JMU connate water with the
Mule Shoe water on this chart for the 8.4, 9.5, and 10.5 pH values, as
shown in Figure 13-6-A, B, and C, respectively. Figure 13-6 indicates
that illite becomes less stable at higher pH. He also investigated the effect
of the K + activity on the illite stability. The mixing curves for 0, 2
and 5 weight % KCl solutions at the 10.5 pH level are shown in Figures
13-7-A, B, and C, respectively. Clearly, adding KCl increases the illite
stability. However, K + activity has a relatively smaller effect than pH,
in view of the comparison of Figures 13-6 and 13-7.