Page 244 - Safety Risk Management for Medical Devices
P. 244
Risk Management and Product Development Process 223
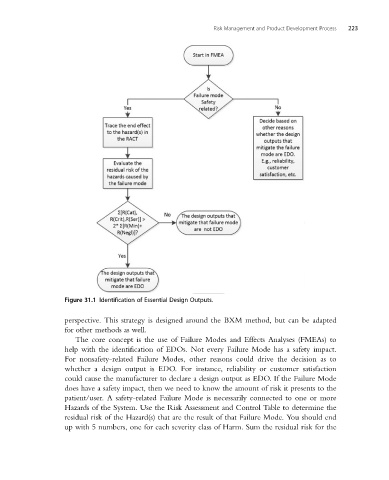
Figure 31.1 Identification of Essential Design Outputs.
perspective. This strategy is designed around the BXM method, but can be adapted
for other methods as well.
The core concept is the use of Failure Modes and Effects Analyses (FMEAs) to
help with the identification of EDOs. Not every Failure Mode has a safety impact.
For nonsafety-related Failure Modes, other reasons could drive the decision as to
whether a design output is EDO. For instance, reliability or customer satisfaction
could cause the manufacturer to declare a design output as EDO. If the Failure Mode
does have a safety impact, then we need to know the amount of risk it presents to the
patient/user. A safety-related Failure Mode is necessarily connected to one or more
Hazards of the System. Use the Risk Assessment and Control Table to determine the
residual risk of the Hazard(s) that are the result of that Failure Mode. You should end
up with 5 numbers, one for each severity class of Harm. Sum the residual risk for the