Page 98 - Theory and Problems of BEGINNING CHEMISTRY
P. 98
CHAP. 6] INORGANIC NOMENCLATURE 87
Start
Yes No
Any
(Sec. 6.3) ions?
Name cation
Yes Binary No
nonmetal?
Yes No
Monatomic
cation?
(Sec. 6.2)
See Table 6-3 Name leftmost or
lower element: then
Yes Only one No use prefix plus stem
of other element
cation for
element? plus -ide
Use element
Use name plus
element roman numeral
name for charge
Yes
Acid?
(Sec. 6.4)
Name anion
Special Special, Oxyanion
simple, or
Start
oxyanion?
Simple
Still
Monatomic fewer Fewer Most More
anion O atoms common O atoms
See Table 6-6 O atoms
—ide hypo—ite —ite —ate per—ate
hydro—ic hypo—ous —ous —ic per—ic
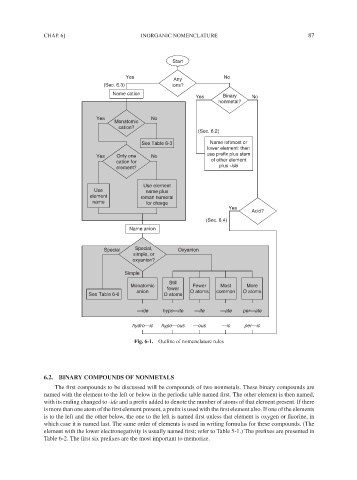
Fig. 6-1. Outline of nomenclature rules
6.2. BINARY COMPOUNDS OF NONMETALS
The first compounds to be discussed will be compounds of two nonmetals. These binary compounds are
named with the element to the left or below in the periodic table named first. The other element is then named,
with its ending changed to -ide and a prefix added to denote the number of atoms of that element present. If there
is more than one atom of the first element present, a prefix is used with the first element also. If one of the elements
is to the left and the other below, the one to the left is named first unless that element is oxygen or fluorine, in
which case it is named last. The same order of elements is used in writing formulas for these compounds. (The
element with the lower electronegativity is usually named first; refer to Table 5-1.) The prefixes are presented in
Table 6-2. The first six prefixes are the most important to memorize.