Page 46 - Sedimentology and Stratigraphy
P. 46
Limestone 33
present consensus is that they form by chemical pre-
cipitation out of agitated water saturated in calcium
carbonate in warm waters (Tucker & Wright 1990). It
is likely that bacteria also play a role in the process,
especially in less agitated environments (Folk & Lynch
2001). Concentrically layered carbonate particles
over 2 mm across are called pisoids: these are often
more irregular in shape but are otherwise similar in
form and origin to ooids.
Some round particles made up of fine-grained cal-
cium carbonate found in sediments do not show any
concentric structure and have apparently not grown
in water in the same way as an ooid or pisoid. These
peloids are commonly the faecal pellets of marine
organisms such as gastropods and may be very abun-
dant in some carbonate deposits, mostly as particles
less than a millimetre across. In thin-section these
pellets are internally homogeneous, and, if the rock
underwent some early compaction, they may have
become deformed, squashed between harder grains,
making them difficult to distinguish from loose mud
deposited as a matrix.
Intraclasts are fragments of calcium carbonate
material that has been partly lithified and then
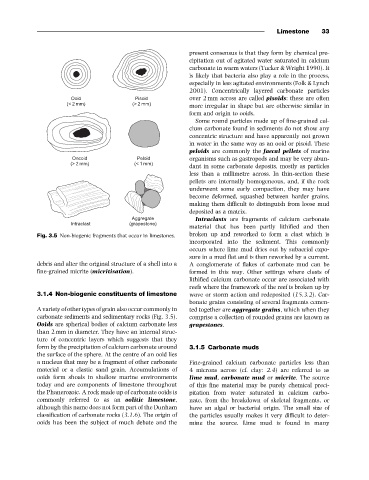
Fig. 3.5 Non-biogenic fragments that occur in limestones. broken up and reworked to form a clast which is
incorporated into the sediment. This commonly
occurs where lime mud dries out by subaerial expo-
sure in a mud flat and is then reworked by a current.
debris and alter the original structure of a shell into a A conglomerate of flakes of carbonate mud can be
fine-grained micrite (micritisation). formed in this way. Other settings where clasts of
lithified calcium carbonate occur are associated with
reefs where the framework of the reef is broken up by
3.1.4 Non-biogenic constituents of limestone wave or storm action and redeposited (15.3.2). Car-
bonate grains consisting of several fragments cemen-
A variety of other types of grain also occur commonly in ted together are aggregate grains, which when they
carbonate sediments and sedimentary rocks (Fig. 3.5). comprise a collection of rounded grains are known as
Ooids are spherical bodies of calcium carbonate less grapestones.
than 2 mm in diameter. They have an internal struc-
ture of concentric layers which suggests that they
form by the precipitation of calcium carbonate around 3.1.5 Carbonate muds
the surface of the sphere. At the centre of an ooid lies
a nucleus that may be a fragment of other carbonate Fine-grained calcium carbonate particles less than
material or a clastic sand grain. Accumulations of 4 microns across (cf. clay: 2.4) are referred to as
ooids form shoals in shallow marine environments lime mud, carbonate mud or micrite. The source
today and are components of limestone throughout of this fine material may be purely chemical preci-
the Phanerozoic. A rock made up of carbonate ooids is pitation from water saturated in calcium carbo-
commonly referred to as an oolitic limestone, nate, from the breakdown of skeletal fragments, or
although this name does not form part of the Dunham have an algal or bacterial origin. The small size of
classification of carbonate rocks (3.1.6). The origin of the particles usually makes it very difficult to deter-
ooids has been the subject of much debate and the mine the source. Lime mud is found in many