Page 49 - Sedimentology and Stratigraphy
P. 49
36 Biogenic, Chemical and Volcanogenic Sediments
5 " 6 3
5 " %
3
954" # 3
- #
2 #
3
757"
) 8
, .
.
$
9 " :
3 44" %
%
,
/
0
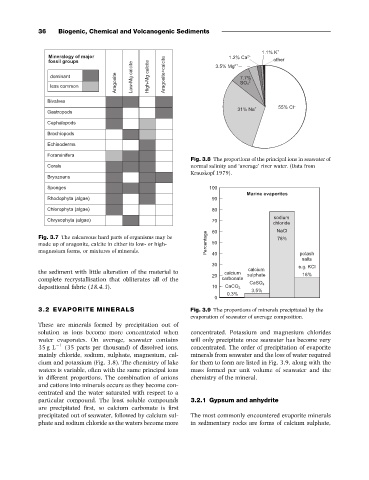
Fig. 3.8 The proportions of the principal ions in seawater of
%
normal salinity and ‘average’ river water. (Data from
Krauskopf 1979).
,
&
) !!
1
=!
%
<!
%
7!
4!
7<"
Fig. 3.7 The calcareous hard parts of organisms may be ;! :
%
made up of aragonite, calcite in either its low- or high-
magnesium forms, or mixtures of minerals. 8!
9! 5 5 6%
the sediment with little alteration of the material to
!
<"
complete recrystallisation that obliterates all of the
'
%
) 8
depositional fabric (18.4.3). ! %
% 9 954"
!59"
!
3.2 EVAPORITE MINERALS Fig. 3.9 The proportions of minerals precipitated by the
evaporation of seawater of average composition.
These are minerals formed by precipitation out of
solution as ions become more concentrated when concentrated. Potassium and magnesium chlorides
water evaporates. On average, seawater contains will only precipitate once seawater has become very
35 g L 1 (35 parts per thousand) of dissolved ions, concentrated. The order of precipitation of evaporite
mainly chloride, sodium, sulphate, magnesium, cal- minerals from seawater and the loss of water required
cium and potassium (Fig. 3.8). The chemistry of lake for them to form are listed in Fig. 3.9, along with the
waters is variable, often with the same principal ions mass formed per unit volume of seawater and the
in different proportions. The combination of anions chemistry of the mineral.
and cations into minerals occurs as they become con-
centrated and the water saturated with respect to a
particular compound. The least soluble compounds 3.2.1 Gypsum and anhydrite
are precipitated first, so calcium carbonate is first
precipitated out of seawater, followed by calcium sul- The most commonly encountered evaporite minerals
phate and sodium chloride as the waters become more in sedimentary rocks are forms of calcium sulphate,