Page 153 - Separation process engineering
P. 153
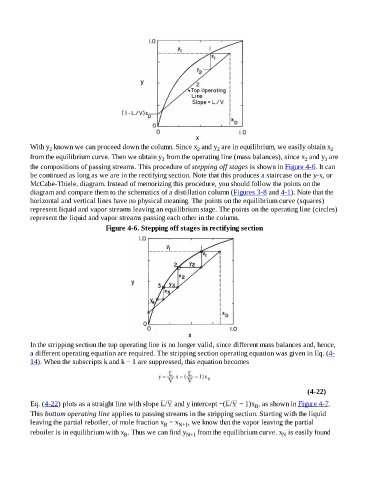
With y known we can proceed down the column. Since x and y are in equilibrium, we easily obtain x 2
2
2
2
from the equilibrium curve. Then we obtain y from the operating line (mass balances), since x and y are
3
2
3
the compositions of passing streams. This procedure of stepping off stages is shown in Figure 4-6. It can
be continued as long as we are in the rectifying section. Note that this produces a staircase on the y-x, or
McCabe-Thiele, diagram. Instead of memorizing this procedure, you should follow the points on the
diagram and compare them to the schematics of a distillation column (Figures 3-8 and 4-1). Note that the
horizontal and vertical lines have no physical meaning. The points on the equilibrium curve (squares)
represent liquid and vapor streams leaving an equilibrium stage. The points on the operating line (circles)
represent the liquid and vapor streams passing each other in the column.
Figure 4-6. Stepping off stages in rectifying section
In the stripping section the top operating line is no longer valid, since different mass balances and, hence,
a different operating equation are required. The stripping section operating equation was given in Eq. (4-
14). When the subscripts k and k − 1 are suppressed, this equation becomes
(4-22)
Eq. (4-22) plots as a straight line with slope / and y intercept −( / − 1)x , as shown in Figure 4-7.
B
This bottom operating line applies to passing streams in the stripping section. Starting with the liquid
leaving the partial reboiler, of mole fraction x = x N+1 , we know that the vapor leaving the partial
B
reboiler is in equilibrium with x . Thus we can find y from the equilibrium curve. x is easily found
B N+1 N