Page 79 - Separation process engineering
P. 79
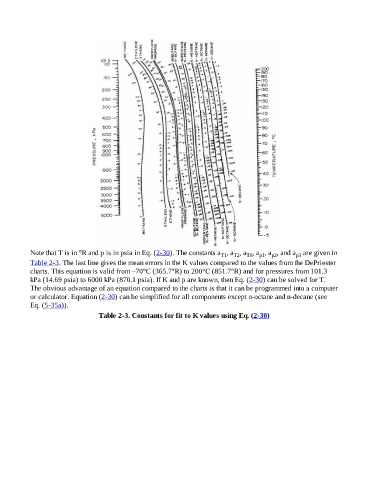
Note that T is in °R and p is in psia in Eq. (2-30). The constants a , a , a , a , a , and a are given in
p2
p3
T1
T6
T2
p1
Table 2-3. The last line gives the mean errors in the K values compared to the values from the DePriester
charts. This equation is valid from –70°C (365.7°R) to 200°C (851.7°R) and for pressures from 101.3
kPa (14.69 psia) to 6000 kPa (870.1 psia). If K and p are known, then Eq. (2-30) can be solved for T.
The obvious advantage of an equation compared to the charts is that it can be programmed into a computer
or calculator. Equation (2-30) can be simplified for all components except n-octane and n-decane (see
Eq. (5-35a)).
Table 2-3. Constants for fit to K values using Eq. (2-30)