Page 34 - Standard Handbook Petroleum Natural Gas Engineering VOLUME2
P. 34
24 Reservoir Engineering
IO
Temperature, F.
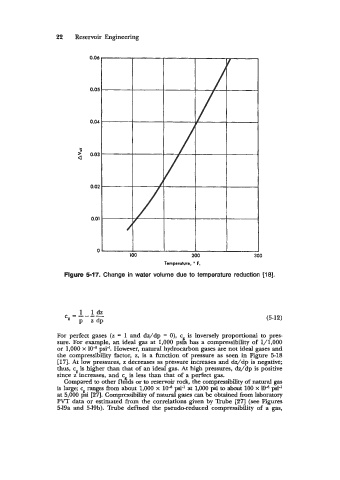
Figure 5-17. Change in water volume due to temperature reduction [18].
(5-12)
For perfect gases (z = 1 and dz/dp = 0), cs is inversely proportional to pres-
sure. For example, an ideal gas at 1,000 psia has a compressibility of 1/1,000
or 1,000 x psi-’. However, natural hydrocarbon gases are not ideal gases and
the compressibility factor, z, is a function of pressure as seen in Figure 5-18
[17]. At low pressures, z decreases as pressure increases and dz/dp is negative;
thus, cg is higher than that of an ideal gas. At high pressures, dz/dp is positive
since z increases, and cs is less than that of a perfect gas.
Compared to other flwds or to reservoir rock, the compressibility of natural gas
is large; cg ranges from about 1,000 x psi-’ at 1,000 psi to about 100 x psi-’
at 5,000 psi [227]. Compressibility of natural gases can be obtained from laboratory
PVT data or estimated from the correlations given by Trube [27] (see Figures
5-19a and 5-19b). Trube defined the pseudo-reduced compressibility of a gas,