Page 452 - Standard Handbook Petroleum Natural Gas Engineering VOLUME2
P. 452
412 Production
where SA and T, are the gravity and boiling point of the hypothetical n-alkane
of the M of the substance of interest and are given by Equation 6-31.
The coefficients Ai in Equation 6-320 are given by
A, = ai + bM
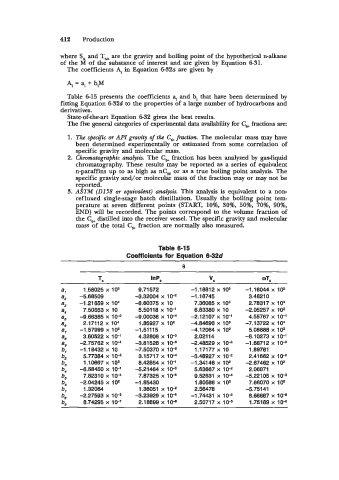
Table 6-15 presents the coefficients a, and bi that have been determined by
fitting Equation 6-821 to the properties of a large number of hydrocarbons and
derivatives.
State-of-the-art Equation 6-32 gives the best results.
The five general categories of experimental data availability for C, fractions are:
1. The specific or API pvity of the C,@ction. The molecular mass may have
been determined experimentally or estimated from some correlation of
specific gravity and molecular mass.
2. Chromatographic analysis. The C, fraction has been analyzed by gas-liquid
chromatography. These results may be reported as a series of equivalent
n-paraffins up to as high as nC,, or as a true boiling point analysis. The
specific gravity and/or molecular mass of the fraction may or may not be
reported.
3. ASTM (0158 or equivalent) analysis. This analysis is equivalent to a non-
refluxed single-stage batch distillation. Usually the boiling point tem-
perature at seven different points (START, 1096, 3096, 5096, 7096, 9096,
END) will be recorded. The points correspond to the volume fraction of
the C, distilled into the receiver vessel. The specific gravity and molecular
mass of the total C, fraction are normally also measured.
Table 6-15
Coefficients for Equation 6-32d
e
Te InP, "e WTe
a, 1.58025 x 10s 9.71 572 -1.18812 x 108 -1.16044 x 10s
a, -5.68509 -3.32004 x lo+ -1.18745 3.4821 0
as -1.21659 x lo4 -8.60375 x 10 7.36085 x lo3 2.78317 x lo4
ar 7.50653 x 10 5.50118 x 10-l 6.83380 x 10 -2.05257 x lo2
a, -9.66385 x lo4 -9.00036 x 1V -2.12107 x lV1 4.55767 x 10-l
ag 2.17112 X lo4 1.85927 x 102 4.84696 x 10' -7.13722 X lo4
a, -1.57999 x lo2 -1.5111 5 4.12064 x lo2 5.08888 x lo2
a, 3.60522 x 10-1 4.32808 x 109 2.02114 -6.10273 x 10-l
a, -2.75762 x 10-4 -3.81526 x 1V -2.48529 X 109 -1.68712 X 10-9
b, -1.18432 x 10 -7.50370 x 1V 1.17177 x 10 1 .E9761
b, 5.77384 x 10-2 3.15717 x 1W -3.48927 x lo+ 2.41662 x 10-2
b, 1 .lo697 x lo2 8.42854 x lo-' -1.34146 x 102 -2.67462 x 102
b, -6.58450 x 1V' -5.21464 X 10-9 5.63667 x lo4 2.06071
b5 7.82310 x lW 7.87325 x lod 9.52631 x 10-4 -5.22105 x 109
b, -2.04245 x lo2 -1 .E5430 1.80586 x lo2 7.66070 x lo2
b, 1.32064 1.36051 x 1 O4 2.56478 -5.75141
be -2.27593 x 109 -3.23929 x 106 -1.74431 X 10-2 8.66667 X lo4
b, 8.74295 x lo-' 2.18899 x lod 2.50717 x 106 1.75189 x 106