Page 203 - Strategies and Applications in Quantum Chemistry From Molecular Astrophysics to Molecular Engineer
P. 203
186 Y. G. SMEYERS ET AL.
In Table 5, we give the HPHF energy values obtained for the singlet ground state,
and the first triplet and singlet excited states, as well as the RHF and UHF values
obtained elsewhere [20] with different basis sets. It is seen that whereas con-
formation is the preferred in the singlet ground state, the one is the most
stable in the excited states in accordance with the large band progressions observed
in the electronic spectrum, as well as the band assignments [20]. This change of
conformational preference can be also interpreted on the basis of the destabilization
of the electronic system.
Here, once more the barrier height values are seen to be very basis dependent. The
value encountered for the singlet excited state, however, is found to be in relatively
good agreement with the experimental value:
3.4. FORMIC ACID
Finally, the HPHF approach is applied to the formic acid molecule, in its first singlet
excited state, which is not orthogonal any more to the singlet ground state in a
random conformation.
The calculations were performed into two basis sets, with full geometry optimization
except for the torsional angles and Two non planar conformations were con-
sidered, which correspond to minima on the potential energy surface into the GVB
approximation [21]. In these conformations, the molecule adopts a pyramidal confor-
mation, as in methanal. In addition, the hydroxilic group is rotated up or down the
OCO plane.
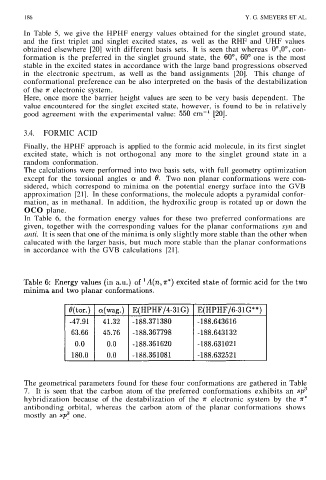
In Table 6, the formation energy values for these two preferred conformations are
given, together with the corresponding values for the planar conformations syn and
anti. It is seen that one of the minima is only slightly more stable than the other when
calucated with the larger basis, but much more stable than the planar conformations
in accordance with the GVB calculations [21].
The geometrical parameters found for these four conformations are gathered in Table
7. It is seen that the carbon atom of the preferred conformations exhibits an
hybridization because of the destabilization of the electronic system by the
antibonding orbital, whereas the carbon atom of the planar conformations shows
mostly an one.