Page 210 - Strategies and Applications in Quantum Chemistry From Molecular Astrophysics to Molecular Engineer
P. 210
FSGO HARTREE-FOCK INSTABILITIES OF HYDROGEN 193
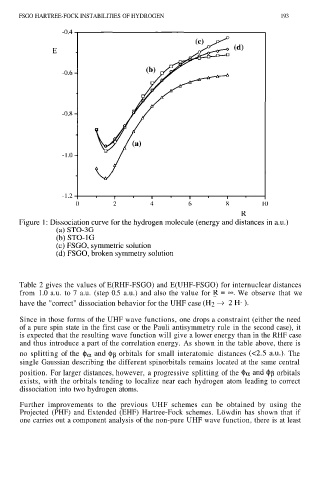
Table 2 gives the values of E(RHF-FSGO) and E(UHF-FSGO) for internuclear distances
from 1.0 a.u. to 7 a.u. (step 0.5 a.u.) and also the value for We observe that we
have the "correct" dissociation behavior for the UHF case
Since in those forms of the UHF wave functions, one drops a constraint (either the need
of a pure spin state in the first case or the Pauli antisymmetry rule in the second case), it
is expected that the resulting wave function will give a lower energy than in the RHF case
and thus introduce a part of the correlation energy. As shown in the table above, there is
no splitting of the orbitals for small interatomic distances The
single Gaussian describing the different spinorbitals remains located at the same central
position. For larger distances, however, a progressive splitting of the orbitals
exists, with the orbitals tending to localize near each hydrogen atom leading to correct
dissociation into two hydrogen atoms.
Further improvements to the previous UHF schemes can be obtained by using the
Projected (PHF) and Extended (EHF) Hartree-Fock schemes. Löwdin has shown that if
one carries out a component analysis of the non-pure UHF wave function, there is at least