Page 211 - Strategies and Applications in Quantum Chemistry From Molecular Astrophysics to Molecular Engineer
P. 211
194 J. M. ANDRÉ ET AL.
one component which restores the symmetry and which has a lower energy than the UHF
wave function.
The form of the PHF wave function is, in this case:
Note that the PHF wave function is no longer a single determinant and is a sum of two
terms. This PHF function both satisfies the Pauli principle and is a pure singlet state. The
energy formula E(PHF-FSGO) is easily derived:
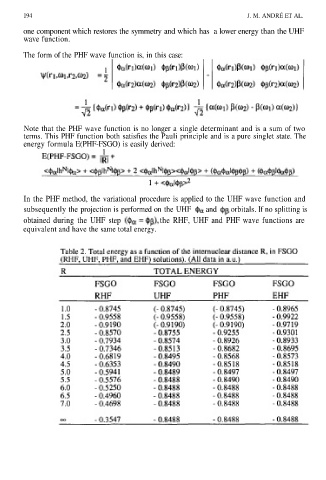
In the PHF method, the variational procedure is applied to the UHF wave function and
subsequently the projection is performed on the UHF and orbitals. If no splitting is
obtained during the UHF step the RHF, UHF and PHF wave functions are
equivalent and have the same total energy.