Page 273 - Strategies and Applications in Quantum Chemistry From Molecular Astrophysics to Molecular Engineer
P. 273
256 V. BARONE ET AL.
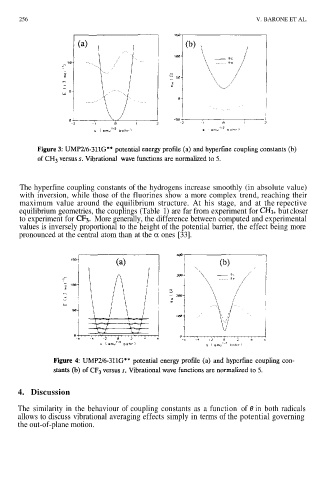
The hyperfine coupling constants of the hydrogens increase smoothly (in absolute value)
with inversion, while those of the fluorines show a more complex trend, reaching their
maximum value around the equilibrium structure. At his stage, and at the repective
equilibrium geometries, the couplings (Table 1) are far from experiment for butcloser
to experiment for More generally, the difference between computed and experimental
values is inversely proportional to the height of the potential barrier, the effect being more
pronounced at the central atom than at the α ones [33].
4. Discussion
The similarity in the behaviour of coupling constants as a function of in both radicals
allows to discuss vibrational averaging effects simply in terms of the potential governing
the out-of-plane motion.